Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding
- PMID: 17989695
- PMCID: PMC2099469
- DOI: 10.1038/sj.emboj.7601906
Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding
Abstract
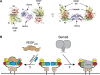
Neuropilins (Nrps) are co-receptors for class 3 semaphorins and vascular endothelial growth factors and important for the development of the nervous system and the vasculature. The extracellular portion of Nrp is composed of two domains that are essential for semaphorin binding (a1a2), two domains necessary for VEGF binding (b1b2), and one domain critical for receptor dimerization (c). We report several crystal structures of Nrp1 and Nrp2 fragments alone and in complex with antibodies that selectively block either semaphorin or vascular endothelial growth factor (VEGF) binding. In these structures, Nrps adopt an unexpected domain arrangement in which the a2, b1, and b2 domains form a tightly packed core that is only loosely connected to the a1 domain. The locations of the antibody epitopes together with in vitro experiments indicate that VEGF and semaphorin do not directly compete for Nrp binding. Based upon our structural and functional data, we propose possible models for ligand binding to neuropilins.
Figures

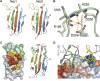

References
-
- Antipenko A, Himanen JP, van Leyen K, Nardi-Dei V, Lesniak J, Barton WA, Rajashankar KR, Lu M, Hoemme C, Puschel AW, Nikolov DB (2003) Structure of the semaphorin-3A receptor binding module. Neuron 39: 589–598 - PubMed
-
- Beckmann G, Bork P (1993) An adhesive domain detected in functionally diverse receptors. Trends Biochem Sci 18: 40–41 - PubMed
-
- Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, Lowenstein DH, Skarnes WC, Chedotal A, Tessier-Lavigne M (2000) Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 25: 43–56 - PubMed
-
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M (1997) Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19: 547–559 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

