Preventing serpin aggregation: the molecular mechanism of citrate action upon antitrypsin unfolding
- PMID: 18780818
- PMCID: PMC2590919
- DOI: 10.1110/ps.037234.108
Preventing serpin aggregation: the molecular mechanism of citrate action upon antitrypsin unfolding
Abstract
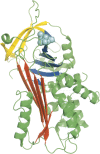
The aggregation of antitrypsin into polymers is one of the causes of neonatal hepatitis, cirrhosis, and emphysema. A similar reaction resulting in disease can occur in other human serpins, and collectively they are known as the serpinopathies. One possible therapeutic strategy involves inhibiting the conformational changes involved in antitrypsin aggregation. The citrate ion has previously been shown to prevent antitrypsin aggregation and maintain the protein in an active conformation; its mechanism of action, however, is unknown. Here we demonstrate that the citrate ion prevents the initial misfolding of the native state to a polymerogenic intermediate in a concentration-dependent manner. Furthermore, we have solved the crystal structure of citrate bound to antitrypsin and show that a single citrate molecule binds in a pocket between the A and B beta-sheets, a region known to be important in maintaining antitrypsin stability.
Figures


References
-
- Bird P.I., Pak S.C., Worrall D.M., Bottomley S.P. Production of recombinant serpins in Escherichia coli . Methods. 2004;32:169–176. - PubMed
-
- Bottomley S.P., Stone S.R. Protein engineering of chimeric serpins: An investigation into effects of the serpin scaffold and reactive centre loop length. Protein Eng. 1998;11:1243–1247. - PubMed
-
- Cabrita L.D., Dai W., Bottomley S.P. Different conformational changes within the F-helix occur during serpin folding, polymerization, and proteinase inhibition. Biochemistry. 2004;43:9834–9839. - PubMed
-
- Carrell R.W., Lomas D.A. Conformational disease. Lancet. 1997;350:134–138. - PubMed
-
- Chow M.K., Devlin G.L., Bottomley S.P. Osmolytes as modulators of conformational changes in serpins. Biol. Chem. 2001;382:1593–1599. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

