Crystal structure of the non-heme iron dioxygenase PtlH in pentalenolactone biosynthesis
- PMID: 17942405
- PMCID: PMC3010413
- DOI: 10.1074/jbc.M706358200
Crystal structure of the non-heme iron dioxygenase PtlH in pentalenolactone biosynthesis
Abstract
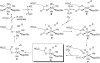
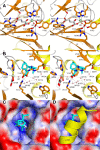
The non-heme iron dioxygenase PtlH from the soil organism Streptomyces avermitilis is a member of the iron(II)/alpha-ketoglutarate-dependent dioxygenase superfamily and catalyzes an essential reaction in the biosynthesis of the sesquiterpenoid antibiotic pentalenolactone. To investigate the structural basis for substrate recognition and catalysis, we have determined the x-ray crystal structure of PtlH in several complexes with the cofactors iron, alpha-ketoglutarate, and the non-reactive enantiomer of the substrate, ent-1-deoxypentalenic acid, in four different crystal forms to up to 1.31 A resolution. The overall structure of PtlH forms a double-stranded barrel helix fold, and the cofactor-binding site for iron and alpha-ketoglutarate is similar to other double-stranded barrel helix fold enzymes. Additional secondary structure elements that contribute to the substrate-binding site in PtlH are not conserved in other double-stranded barrel helix fold enzymes. Binding of the substrate enantiomer induces a reorganization of the monoclinic crystal lattice leading to a disorder-order transition of a C-terminal alpha-helix. The newly formed helix blocks the major access to the active site and effectively traps the bound substrate. Kinetic analysis of wild type and site-directed mutant proteins confirms a critical function of two arginine residues in substrate binding, while simulated docking of the enzymatic reaction product reveals the likely orientation of bound substrate.
Figures
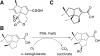

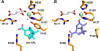

References
-
- Koe BK, Sobin BA, Celmer WD. Antibiot. Annu. 1957:672–675. - PubMed
-
- Keller-Schierlein W, Lemke J, Nyfeler R, Zahner H. Arch. Mikrobiol. 1972;84:301–316. - PubMed
-
- Okazaki T, Enokita R, Torikata A, Inukai M, Takeuchi M, Takahashi S, Arai M. Ann. Rep. Sankyo Res. Lab. 1979;31:94–103.
-
- Takahashi S, Takeuchi M, Arai M, Seto H, Otake N. J Antibiot. 1983;36(3):226–228. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

