Natural beta-sheet proteins use negative design to avoid edge-to-edge aggregation
- PMID: 11880627
- PMCID: PMC122420
- DOI: 10.1073/pnas.052706099
Natural beta-sheet proteins use negative design to avoid edge-to-edge aggregation
Abstract
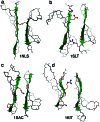
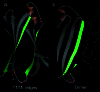
The fact that natural beta-sheet proteins are usually soluble but that fragments or designs of beta structure usually aggregate suggests that natural beta proteins must somehow be designed to avoid this problem. Regular beta-sheet edges are dangerous, because they are already in the right conformation to interact with any other beta strand they encounter. We surveyed edge strands in a large sample of all-beta proteins to tabulate features that could protect against further beta-sheet interactions. beta-barrels, of course, avoid edges altogether by continuous H-bonding around the barrel cylinder. Parallel beta-helix proteins protect their beta-sheet ends by covering them with loops of other structure. beta-propeller and single-sheet proteins use a combination of beta-bulges, prolines, strategically placed charges, very short edge strands, and loop coverage. beta-sandwich proteins favor placing an inward-pointing charged side chain on one of the edge strands where it would be buried by dimerization; they also use bulges, prolines, and other mechanisms. One recent beta-hairpin design has a constrained twist too great for accommodation into a larger beta-sheet, whereas some beta-sheet edges are protected by the bend and reverse twist produced by an Lbeta glycine. All free edge strands were seen to be protected, usually by several redundant mechanisms. In contrast, edge strands that natively form beta H-bonded dimers or rings have long, regular stretches without such protection. These results are relevant to understanding how proteins may assemble into beta-sheet amyloid fibers, and they are especially applicable to the de novo design of beta structure. Many edge-protection strategies used by natural proteins are beyond our current abilities to constrain by design, but one possibility stands out as especially useful: a single charged side chain near the middle of what would ordinarily be the hydrophobic side of the edge beta strand. This minimal negative-design strategy changes only one residue, requires no backbone distortion, and is easy to design. The accompanying paper [Wang, W. & Hecht, M. H. (2002) Proc. Natl. Acad. Sci. USA 99, 2760-2765] makes use of the inward-pointing charge strategy with great success, turning highly aggregated beta-sandwich designs into soluble monomers.
Figures
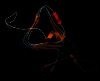



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

