Thioredoxin A active-site mutants form mixed disulfide dimers that resemble enzyme-substrate reaction intermediates
- PMID: 18455736
- PMCID: PMC2896474
- DOI: 10.1016/j.jmb.2008.03.077
Thioredoxin A active-site mutants form mixed disulfide dimers that resemble enzyme-substrate reaction intermediates
Abstract
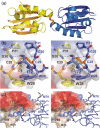
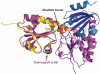
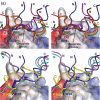
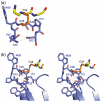
Thioredoxin functions in nearly all organisms as the major thiol-disulfide oxidoreductase within the cytosol. Its prime purpose is to maintain cysteine-containing proteins in the reduced state by converting intramolecular disulfide bonds into dithiols in a disulfide exchange reaction. Thioredoxin has been reported to contribute to a wide variety of physiological functions by interacting with specific sets of substrates in different cell types. To investigate the function of the essential thioredoxin A (TrxA) in the low-GC Gram-positive bacterium Bacillus subtilis, we purified wild-type TrxA and three mutant TrxA proteins that lack either one or both of the two cysteine residues in the CxxC active site. The pure proteins were used for substrate-binding studies known as "mixed disulfide fishing" in which covalent disulfide-bonded reaction intermediates can be visualized. An unprecedented finding is that both active-site cysteine residues can form mixed disulfides with substrate proteins when the other active-site cysteine is absent, but only the N-terminal active-site cysteine forms stable interactions. A second novelty is that both single-cysteine mutant TrxA proteins form stable homodimers due to thiol oxidation of the remaining active-site cysteine residue. To investigate whether these dimers resemble mixed enzyme-substrate disulfides, the structure of the most abundant dimer, C32S, was characterized by X-ray crystallography. This yielded a high-resolution (1.5A) X-ray crystallographic structure of a thioredoxin homodimer from a low-GC Gram-positive bacterium. The C32S TrxA dimer can be regarded as a mixed disulfide reaction intermediate of thioredoxin, which reveals the diversity of thioredoxin/substrate-binding modes.
Figures


References
-
- Arner E.S., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. - PubMed
-
- Laurent T.C., Moore E.C., Richard P. Enzymatic synthesis of deoxyribonucleotides: IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J. Biol. Chem. 1964;239:3436–3444. - PubMed
-
- Powis G., Mustacich D., Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic. Biol. Med. 2000;29:312–322. - PubMed
-
- Schurmann P. Redox signaling in the chloroplast: the ferredoxin/thioredoxin system. Antioxid. Redox Signal. 2003;5:69–78. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

