Cross-link formation of the cysteine 228-tyrosine 272 catalytic cofactor of galactose oxidase does not require dioxygen
- PMID: 18771294
- PMCID: PMC2762401
- DOI: 10.1021/bi8010835
Cross-link formation of the cysteine 228-tyrosine 272 catalytic cofactor of galactose oxidase does not require dioxygen
Abstract
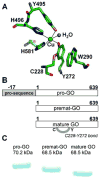
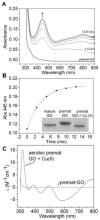
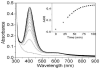
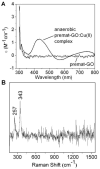
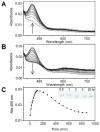
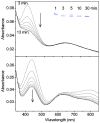
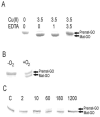
Galactose oxidase (GO) belongs to a class of proteins that self-catalyze assembly of their redox-active cofactors from active site amino acids. Generation of enzymatically active GO appears to require at least four sequential post-translational modifications: cleavage of a secretion signal sequence, copper-dependent cleavage of an N-terminal pro sequence, copper-dependent formation of a C228-Y272 thioether bond, and generation of the Y272 radical. The last two processes were investigated using a truncated protein (termed premat-GO) lacking the pro sequence and purified under copper-free conditions. Reactions of premat-GO with Cu(II) were investigated using optical, EPR, and resonance Raman spectroscopy, SDS-PAGE, and X-ray crystallography. Premat-GO reacted anaerobically with excess Cu(II) to efficiently form the thioether bond but not the Y272 radical. A potential C228-copper coordinated intermediate (lambda max = 406 nm) in the processing reaction, which had not yet formed the C228-Y272 cross-link, was identified from the absorption spectrum. A copper-thiolate protein complex, with copper coordinated to C228, H496, and H581, was also observed in a 3 min anaerobic soak by X-ray crystallography, whereas a 24 h soak revealed the C228-Y272 thioether bond. In solution, addition of oxygenated buffer to premat-GO preincubated with excess Cu(II) generated the Y272 radical state. On the basis of these data, a mechanism for the formation of the C228-Y272 bond and tyrosyl radical generation is proposed. The 406 nm complex is demonstrated to be a catalytically competent processing intermediate under anaerobic conditions. We propose a potential mechanism which is in common with aerobic processing by Cu(II) until the step at which the second electron acceptor is required.
Figures


References
-
- Okeley NM, van der Donk WA. Novel cofactors via post-translational modifications of enzyme active sites. Chem Biol. 2000;7:R159–R171. - PubMed
-
- Stubbe JA. Protein radical involvement in biological catalysis. Annu Rev Biochem. 1989;58:257–285. - PubMed
-
- Stubbe JA, van der Donk WA. Protein radicals in enzyme catalysis. Chem ReV. 1998;98:705–762. - PubMed
-
- Frey PA. Radical mechanisms of enzymatic catalysis. Annu Rev Biochem. 2001;70:121–148. - PubMed
-
- Banerjee R. Introduction: Radical enzymology. Chem Rev. 2003;103:2081–2081.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

