Structure and function of a complex between chorismate mutase and DAHP synthase: efficiency boost for the junior partner
- PMID: 19556970
- PMCID: PMC2718287
- DOI: 10.1038/emboj.2009.165
Structure and function of a complex between chorismate mutase and DAHP synthase: efficiency boost for the junior partner
Abstract
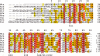
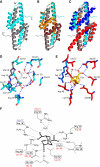
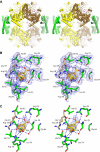
Chorismate mutase catalyzes a key step in the shikimate biosynthetic pathway towards phenylalanine and tyrosine. Curiously, the intracellular chorismate mutase of Mycobacterium tuberculosis (MtCM; Rv0948c) has poor activity and lacks prominent active-site residues. However, its catalytic efficiency increases >100-fold on addition of DAHP synthase (MtDS; Rv2178c), another shikimate-pathway enzyme. The 2.35 A crystal structure of the MtCM-MtDS complex bound to a transition-state analogue shows a central core formed by four MtDS subunits sandwiched between two MtCM dimers. Structural comparisons imply catalytic activation to be a consequence of the repositioning of MtCM active-site residues on binding to MtDS. The mutagenesis of the C-terminal extrusion of MtCM establishes conserved residues as part of the activation machinery. The chorismate-mutase activity of the complex, but not of MtCM alone, is inhibited synergistically by phenylalanine and tyrosine. The complex formation thus endows the shikimate pathway of M. tuberculosis with an important regulatory feature. Experimental evidence suggests that such non-covalent enzyme complexes comprising an AroQ(delta) subclass chorismate mutase like MtCM are abundant in the bacterial order Actinomycetales.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



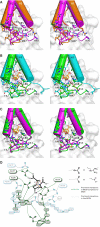

References
-
- Ahmad S, Jensen RA (1986) The evolutionary history of two bifunctional proteins that emerged in the purple bacteria. Trends Biochem Sci 11: 108–112
-
- Andrews PR, Smith GD, Young IG (1973) Transition-state stabilization and enzymic catalysis. Kinetic and molecular orbital studies of the rearrangement of chorismate to prephenate. Biochemistry 12: 3492–3498 - PubMed
-
- Bartlett PA, Johnson CR (1985) An inhibitor of chorismate mutase resembling the transition-state conformation. J Am Chem Soc 107: 7792–7793
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

