Structural insight into nascent polypeptide chain-mediated translational stalling
- PMID: 19933110
- PMCID: PMC2920484
- DOI: 10.1126/science.1177662
Structural insight into nascent polypeptide chain-mediated translational stalling
Abstract
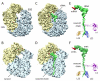
Expression of the Escherichia coli tryptophanase operon depends on ribosome stalling during translation of the upstream TnaC leader peptide, a process for which interactions between the TnaC nascent chain and the ribosomal exit tunnel are critical. We determined a 5.8 angstrom-resolution cryo-electron microscopy and single-particle reconstruction of a ribosome stalled during translation of the tnaC leader gene. The nascent chain was extended within the exit tunnel, making contacts with ribosomal components at distinct sites. Upon stalling, two conserved residues within the peptidyltransferase center adopted conformations that preclude binding of release factors. We propose a model whereby interactions within the tunnel are relayed to the peptidyltransferase center to inhibit translation. Moreover, we show that nascent chains adopt distinct conformations within the ribosomal exit tunnel.
Figures


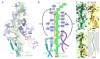
Comment in
-
Biochemistry. Nascent proteins caught in the act.Science. 2009 Dec 4;326(5958):1352-3. doi: 10.1126/science.1183690. Science. 2009. PMID: 19965743 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

