Structural insights into the dual nucleotide exchange and GDI displacement activity of SidM/DrrA
- PMID: 19942850
- PMCID: PMC2824451
- DOI: 10.1038/emboj.2009.347
Structural insights into the dual nucleotide exchange and GDI displacement activity of SidM/DrrA
Abstract
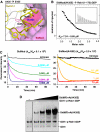
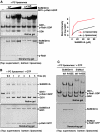
GDP-bound prenylated Rabs, sequestered by GDI (GDP dissociation inhibitor) in the cytosol, are delivered to destined sub-cellular compartment and subsequently activated by GEFs (guanine nucleotide exchange factors) catalysing GDP-to-GTP exchange. The dissociation of GDI from Rabs is believed to require a GDF (GDI displacement factor). Only two RabGDFs, human PRA-1 and Legionella pneumophila SidM/DrrA, have been identified so far and the molecular mechanism of GDF is elusive. Here, we present the structure of a SidM/DrrA fragment possessing dual GEF and GDF activity in complex with Rab1. SidM/DrrA reconfigures the Switch regions of the GTPase domain of Rab1, as eukaryotic GEFs do toward cognate Rabs. Structure-based mutational analyses show that the surface of SidM/DrrA, catalysing nucleotide exchange, is involved in GDI1 displacement from prenylated Rab1:GDP. In comparison with an eukaryotic GEF TRAPP I, this bacterial GEF/GDF exhibits high binding affinity for Rab1 with GDP retained at the active site, which appears as the key feature for the GDF activity of the protein.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Ali BR, Seabra MC (2005) Targeting of Rab GTPases to cellular membranes. Biochem Soc Trans 33(Part 4): 652–656 - PubMed
-
- Allan BB, Moyer BD, Balch WE (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289: 444–448 - PubMed
-
- Araki S, Kikuchi A, Hata Y, Isomura M, Takai Y (1990) Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem 265: 13007–13015 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

