The quorum-quenching N-acyl homoserine lactone acylase PvdQ is an Ntn-hydrolase with an unusual substrate-binding pocket
- PMID: 20080736
- PMCID: PMC2818923
- DOI: 10.1073/pnas.0911839107
The quorum-quenching N-acyl homoserine lactone acylase PvdQ is an Ntn-hydrolase with an unusual substrate-binding pocket
Abstract
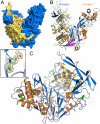
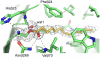
In many Gram-negative pathogens, their virulent behavior is regulated by quorum sensing, in which diffusible signals such as N-acyl homoserine lactones (AHLs) act as chemical messaging compounds. Enzymatic degradation of these diffusible signals by, e.g., lactonases or amidohydrolases abolishes AHL regulated virulence, a process known as quorum quenching. Here we report the first crystal structure of an AHL amidohydrolase, the AHL acylase PvdQ from Pseudomonas aeruginosa. PvdQ has a typical alpha/beta heterodimeric Ntn-hydrolase fold, similar to penicillin G acylase and cephalosporin acylase. However, it has a distinct, unusually large, hydrophobic binding pocket, ideally suited to recognize C12 fatty acid-like chains of AHLs. Binding of a C12 fatty acid or a 3-oxo-C12 fatty acid induces subtle conformational changes to accommodate the aliphatic chain. Furthermore, the structure of a covalent ester intermediate identifies Serbeta1 as the nucleophile and Asnbeta269 and Valbeta70 as the oxyanion hole residues in the AHL degradation process. Our structures show the versatility of the Ntn-hydrolase scaffold and can serve as a structural paradigm for Ntn-hydrolases with similar substrate preference. Finally, the quorum-quenching capabilities of PvdQ may be utilized to suppress the quorum-sensing machinery of pathogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. - PubMed
-
- Eberhard A, et al. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. - PubMed
-
- Whitehead NA, et al. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev. 2001;25:365–404. - PubMed
-
- Dong YH, et al. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001;411:813–817. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

