Molecular mechanism of elongation factor 1A inhibition by a Legionella pneumophila glycosyltransferase
- PMID: 20030628
- PMCID: PMC3518269
- DOI: 10.1042/BJ20091351
Molecular mechanism of elongation factor 1A inhibition by a Legionella pneumophila glycosyltransferase
Abstract
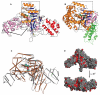
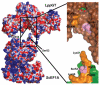
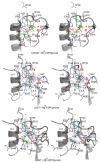
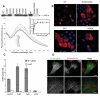
Legionnaires' disease is caused by a lethal colonization of alveolar macrophages with the Gram-negative bacterium Legionella pneumophila. LpGT (L. pneumophila glucosyltransferase; also known as Lgt1) has recently been identified as a virulence factor, shutting down protein synthesis in the human cell by specific glucosylation of EF1A (elongation factor 1A), using an unknown mode of substrate recognition and a retaining mechanism for glycosyl transfer. We have determined the crystal structure of LpGT in complex with substrates, revealing a GT-A fold with two unusual protruding domains. Through structure-guided mutagenesis of LpGT, several residues essential for binding of the UDP-glucose-donor and EF1A-acceptor substrates were identified, which also affected L. pneumophila virulence as demonstrated by microinjection studies. Together, these results suggested that a positively charged EF1A loop binds to a negatively charged conserved groove on the LpGT structure, and that two asparagine residues are essential for catalysis. Furthermore, we showed that two further L. pneumophila glycosyltransferases possessed the conserved UDP-glucose-binding sites and EF1A-binding grooves, and are, like LpGT, translocated into the macrophage through the Icm/Dot (intracellular multiplication/defect in organelle trafficking) system.
Figures

References
-
- Coutinho PM, Henrissat B. Carbohydrate-active enzymes: an integrated database approach. In: Gilbert HJ, Davies G, Henrissat B, Svensson B, editors. Recent Advances in Carbohydrate Bioengineering. The Royal Society of Chemistry; Cambridge: 1999. pp. 3–12.
-
- Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. - PubMed
-
- Schirmer J, Aktories K. Large clostridial cytotoxins: cellular biology of Rho/Ras-glucosylating toxins. Biochim. Biophys. Acta. 2004;1673:66–74. - PubMed
-
- Lyerly D, Wilkins TD. Clostridium difficile. Raven Press; New York: 1995.
-
- Jank T, Giesemann T, Aktories K. Rho-glucosylating Clostridium difficile toxins A and B: new insights into structure and function. Glycobiology. 2007;17:15R–22R. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

