The crystal structure of the signal recognition particle in complex with its receptor
- PMID: 21330537
- PMCID: PMC3758919
- DOI: 10.1126/science.1196473
The crystal structure of the signal recognition particle in complex with its receptor
Abstract
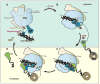
Cotranslational targeting of membrane and secretory proteins is mediated by the universally conserved signal recognition particle (SRP). Together with its receptor (SR), SRP mediates the guanine triphosphate (GTP)-dependent delivery of translating ribosomes bearing signal sequences to translocons on the target membrane. Here, we present the crystal structure of the SRP:SR complex at 3.9 angstrom resolution and biochemical data revealing that the activated SRP:SR guanine triphosphatase (GTPase) complex binds the distal end of the SRP hairpin RNA where GTP hydrolysis is stimulated. Combined with previous findings, these results suggest that the SRP:SR GTPase complex initially assembles at the tetraloop end of the SRP RNA and then relocalizes to the opposite end of the RNA. This rearrangement provides a mechanism for coupling GTP hydrolysis to the handover of cargo to the translocon.
Figures


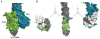


References
-
- Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu Rev Biochem. 2001;70:755. - PubMed
-
- Doudna JA, Batey RT. Structural insights into the signal recognition particle. Annu Rev Biochem. 2004;73:539. - PubMed
-
- Batey RT, Rambo RP, Lucast L, Rha B, Doudna JA. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science. 2000 Feb 18;287:1232. - PubMed
-
- Egea PF, et al. Substrate twinning activates the signal recognition particle and its receptor. Nature. 2004 Jan 15;427:215. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

