Crystal structure of aminomethyltransferase in complex with dihydrolipoyl-H-protein of the glycine cleavage system: implications for recognition of lipoyl protein substrate, disease-related mutations, and reaction mechanism
- PMID: 20375021
- PMCID: PMC2881793
- DOI: 10.1074/jbc.M110.110718
Crystal structure of aminomethyltransferase in complex with dihydrolipoyl-H-protein of the glycine cleavage system: implications for recognition of lipoyl protein substrate, disease-related mutations, and reaction mechanism
Abstract
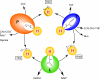
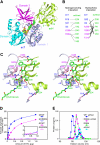
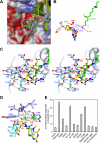
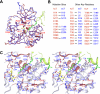
Aminomethyltransferase, a component of the glycine cleavage system termed T-protein, reversibly catalyzes the degradation of the aminomethyl moiety of glycine attached to the lipoate cofactor of H-protein, resulting in the production of ammonia, 5,10-methylenetetrahydrofolate, and dihydrolipoate-bearing H-protein in the presence of tetrahydrofolate. Several mutations in the human T-protein gene are known to cause nonketotic hyperglycinemia. Here, we report the crystal structure of Escherichia coli T-protein in complex with dihydrolipoate-bearing H-protein and 5-methyltetrahydrofolate, a complex mimicking the ternary complex in the reverse reaction. The structure of the complex shows a highly interacting intermolecular interface limited to a small area and the protein-bound dihydrolipoyllysine arm inserted into the active site cavity of the T-protein. Invariant Arg(292) of the T-protein is essential for complex assembly. The structure also provides novel insights in understanding the disease-causing mutations, in addition to the disease-related impairment in the cofactor-enzyme interactions reported previously. Furthermore, structural and mutational analyses suggest that the reversible transfer of the methylene group between the lipoate and tetrahydrofolate should proceed through the electron relay-assisted iminium intermediate formation.
Figures

References
-
- Motokawa Y., Fujiwara K., Okamura-Ikeda K. (1995) in Biothiols in Health and Disease (Packer L., Cadenas E. eds) pp. 389–407, Marcel Dekker, New York
-
- Tada K., Hayasaka K. (1987) Eur. J. Pediatr. 146, 221–227 - PubMed
-
- Nanao K., Okamura-Ikeda K., Motokawa Y., Danks D. M., Baumgartner E. R., Takada G., Hayasaka K. (1994) Hum. Genet. 93, 655–658 - PubMed
-
- Okamura-Ikeda K., Hosaka H., Yoshimura M., Yamashita E., Toma S., Nakagawa A., Fujiwara K., Motokawa Y., Taniguchi H. (2005) J. Mol. Biol. 351, 1146–1159 - PubMed
-
- Okamura-Ikeda K., Fujiwara K., Motokawa Y. (1999) Eur. J. Biochem. 264, 446–452 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

