Human carbonyl reductase 1 is an S-nitrosoglutathione reductase
- PMID: 18826943
- PMCID: PMC2602912
- DOI: 10.1074/jbc.M807125200
Human carbonyl reductase 1 is an S-nitrosoglutathione reductase
Abstract
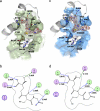
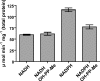
Human carbonyl reductase 1 (hCBR1) is an NADPH-dependent short chain dehydrogenase/reductase with broad substrate specificity and is thought to be responsible for the in vivo reduction of quinones, prostaglandins, and other carbonyl-containing compounds including xenobiotics. In addition, hCBR1 possesses a glutathione binding site that allows for increased affinity toward GSH-conjugated molecules. It has been suggested that the GSH-binding site is near the active site; however, no structures with GSH or GSH conjugates have been reported. We have solved the x-ray crystal structures of hCBR1 and a substrate mimic in complex with GSH and the catalytically inert GSH conjugate hydroxymethylglutathione (HMGSH). The structures reveal the GSH-binding site and provide insight into the affinity determinants for GSH-conjugated substrates. We further demonstrate that the structural isostere of HMGSH, S-nitrosoglutathione, is an ideal hCBR1 substrate (Km = 30 microm, kcat = 450 min(-1)) with kinetic constants comparable with the best known hCBR1 substrates. Furthermore, we demonstrate that hCBR1 dependent GSNO reduction occurs in A549 lung adenocarcinoma cell lysates and suggest that hCBR1 may be involved in regulation of tissue levels of GSNO.
Figures
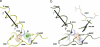

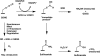
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

