Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices
- PMID: 18611383
- PMCID: PMC2563962
- DOI: 10.1016/j.str.2008.03.017
Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices
Abstract
The human gut microbiota performs functions that are not encoded in our Homo sapiens genome, including the processing of otherwise undigestible dietary polysaccharides. Defining the structures of proteins involved in the import and degradation of specific glycans by saccharolytic bacteria complements genomic analysis of the nutrient-processing capabilities of gut communities. Here, we describe the atomic structure of one such protein, SusD, required for starch binding and utilization by Bacteroides thetaiotaomicron, a prominent adaptive forager of glycans in the distal human gut microbiota. The binding pocket of this unique alpha-helical protein contains an arc of aromatic residues that complements the natural helical structure of starch and imposes this conformation on bound maltoheptaose. Furthermore, SusD binds cyclic oligosaccharides with higher affinity than linear forms. The structures of several SusD/oligosaccharide complexes reveal an inherent ligand recognition plasticity dominated by the three-dimensional conformation of the oligosaccharides rather than specific interactions with the composite sugars.
Figures
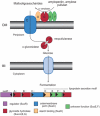

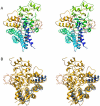
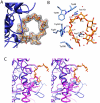
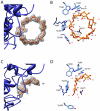

Comment in
-
Sus out sugars in.Structure. 2008 Jul;16(7):987-9. doi: 10.1016/j.str.2008.06.002. Structure. 2008. PMID: 18611370 No abstract available.
References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
-
- Bauer M, Kube M, Teeling H, Richter M, Lombardot T, Allers E, Wurdemann CA, Quast C, Kuhl H, Knaust F, et al. Whole genome analysis of the marine Bacteroidetes‘Gramella forsetii’ reveals adaptations to degradation of polymeric organic matter. Environ Microbiol. 2006;8:2201–2213. - PubMed
-
- Bjursell MK, Martens EC, Gordon JI. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J Biol Chem. 2006;281:36269–36279. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

