Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution
- PMID: 18550812
- PMCID: PMC2449365
- DOI: 10.1073/pnas.0800502105
Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution
Abstract
The hemagglutinin-esterases (HEs) are a family of viral envelope glycoproteins that mediate reversible attachment to O-acetylated sialic acids by acting both as lectins and as receptor-destroying enzymes (RDEs). Related HEs occur in influenza C, toro-, and coronaviruses, apparently as a result of relatively recent lateral gene transfer events. Here, we report the crystal structure of a coronavirus (CoV) HE in complex with its receptor. We show that CoV HE arose from an influenza C-like HE fusion protein (HEF). In the process, HE was transformed from a trimer into a dimer, whereas remnants of the fusion domain were adapted to establish novel monomer-monomer contacts. Whereas the structural design of the RDE-acetylesterase domain remained unaltered, the HE receptor-binding domain underwent remodeling to such extent that the ligand is now bound in opposite orientation. This is surprising, because the architecture of the HEF site was preserved in influenza A HA over a much larger evolutionary distance, a switch in receptor specificity and extensive antigenic variation notwithstanding. Apparently, HA and HEF are under more stringent selective constraints than HE, limiting their exploration of alternative binding-site topologies. We attribute the plasticity of the CoV HE receptor-binding site to evolutionary flexibility conferred by functional redundancy between HE and its companion spike protein S. Our findings offer unique insights into the structural and functional consequences of independent protein evolution after interviral gene exchange and open potential avenues to broad-spectrum antiviral drug design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

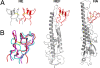

Comment in
-
Viral destruction of cell surface receptors.Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):8807-8. doi: 10.1073/pnas.0804355105. Epub 2008 Jun 23. Proc Natl Acad Sci U S A. 2008. PMID: 18574141 Free PMC article. No abstract available.
References
-
- Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem Rev. 2002;102:439–469. - PubMed
-
- Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: Differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. - PubMed
-
- Shinya K, et al. Avian flu: Influenza virus receptors in the human airway. Nature. 2006;440:435–436. - PubMed
-
- Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. 2002;12:159–166. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

