Golgi alpha-mannosidase II cleaves two sugars sequentially in the same catalytic site
- PMID: 18599462
- PMCID: PMC2474516
- DOI: 10.1073/pnas.0802206105
Golgi alpha-mannosidase II cleaves two sugars sequentially in the same catalytic site
Abstract
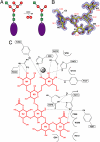
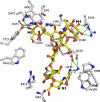
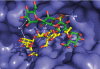
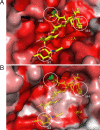
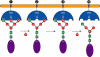
Golgi alpha-mannosidase II (GMII) is a key glycosyl hydrolase in the N-linked glycosylation pathway. It catalyzes the removal of two different mannosyl linkages of GlcNAcMan(5)GlcNAc(2), which is the committed step in complex N-glycan synthesis. Inhibition of this enzyme has shown promise in certain cancers in both laboratory and clinical settings. Here we present the high-resolution crystal structure of a nucleophile mutant of Drosophila melanogaster GMII (dGMII) bound to its natural oligosaccharide substrate and an oligosaccharide precursor as well as the structure of the unliganded mutant. These structures allow us to identify three sugar-binding subsites within the larger active site cleft. Our results allow for the formulation of the complete catalytic process of dGMII, which involves a specific order of bond cleavage, and a major substrate rearrangement in the active site. This process is likely conserved for all GMII enzymes-but not in the structurally related lysosomal mannosidase-and will form the basis for the design of specific inhibitors against GMII.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
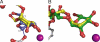
References
-
- Roth J. Protein N-glycosylation along the secretory pathway: Relationship to organelle topography and function, protein quality control, and cell interactions. Chem Rev. 2002;102:285–303. - PubMed
-
- Moremen KW. Golgi α-mannosidase II deficiency in vertebrate systems: Implications for asparagine-linked oligosaccharide processing in mammals. Biochim Biophys Acta. 2002;1573:225–235. - PubMed
-
- Chui D, et al. Alpha-mannosidase-II deficiency results in dyserythropoiesis and unveils an alternate pathway in oligosaccharide biosynthesis. Cell. 1997;90:157–167. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

