Arginine residues at internal positions in a protein are always charged
- PMID: 22080604
- PMCID: PMC3223443
- DOI: 10.1073/pnas.1104808108
Arginine residues at internal positions in a protein are always charged
Abstract
Many functionally essential ionizable groups are buried in the hydrophobic interior of proteins. A systematic study of Lys, Asp, and Glu residues at 25 internal positions in staphylococcal nuclease showed that their pK(a) values can be highly anomalous, some shifted by as many as 5.7 pH units relative to normal pK(a) values in water. Here we show that, in contrast, Arg residues at the same internal positions exhibit no detectable shifts in pK(a); they are all charged at pH ≤ 10. Twenty-three of these 25 variants with Arg are folded at both pH 7 and 10. The mean decrease in thermodynamic stability from substitution with Arg was 6.2 kcal/mol at this pH, comparable to that for substitution with Lys, Asp, or Glu at pH 7. The physical basis behind the remarkable ability of Arg residues to remain protonated in environments otherwise incompatible with charges is suggested by crystal structures of three variants showing how the guanidinium moiety of the Arg side chain is effectively neutralized through multiple hydrogen bonds to protein polar atoms and to site-bound water molecules. The length of the Arg side chain, and slight deformations of the protein, facilitate placement of the guanidinium moieties near polar groups or bulk water. This unique capacity of Arg side chains to retain their charge in dehydrated environments likely contributes toward the important functional roles of internal Arg residues in situations where a charge is needed in the interior of a protein, in a lipid bilayer, or in similarly hydrophobic environments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

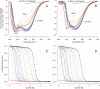

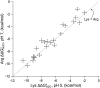
 ) between variants of Δ+PHS SNase with Lys or Arg at internal positions, measured at pH 5 for variants with Lys to ensure they are all charged, and at pH 7 for variants with Arg, where they are all charged. The thermodynamic stability of Δ+PHS SNase is independent of pH between pH 5 and 7 (21). The stability of
) between variants of Δ+PHS SNase with Lys or Arg at internal positions, measured at pH 5 for variants with Lys to ensure they are all charged, and at pH 7 for variants with Arg, where they are all charged. The thermodynamic stability of Δ+PHS SNase is independent of pH between pH 5 and 7 (21). The stability of  values were calculated by subtracting
values were calculated by subtracting  from
from  . The error bars are the estimated uncertainty of the measurement. Values for variants with Lys are from ref. .
. The error bars are the estimated uncertainty of the measurement. Values for variants with Lys are from ref. .References
-
- Bartlett GJ, Porter CT, Borkakoti N, Thornton JM. Analysis of catalytic residues in enzyme active sites. J Mol Biol. 2002;324:105–121. - PubMed
-
- Kim J, Mao J, Gunner MR. Are acidic and basic groups in buried proteins predicted to be ionized? J Mol Biol. 2005;348:1283–1298. - PubMed
-
- Bogan AA, Thorn KS. Anatomy of hot spots in protein interfaces. J Mol Biol. 1998;280:1–9. - PubMed
-
- Cutler RL, et al. Role of arginine-38 in regulation of the cytochrome c oxidation-reduction equilibrium. Biochemistry. 1989;28:3188–3197. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

