The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation
- PMID: 18621668
- PMCID: PMC2527972
- DOI: 10.1126/science.1157987
The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation
Abstract
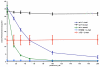
The crystal structure of the high-affinity Escherichia coli MetNI methionine uptake transporter, a member of the adenosine triphosphate (ATP)-binding cassette (ABC) family, has been solved to 3.7 angstrom resolution. The overall architecture of MetNI reveals two copies of the adenosine triphosphatase (ATPase) MetN in complex with two copies of the transmembrane domain MetI, with the transporter adopting an inward-facing conformation exhibiting widely separated nucleotide binding domains. Each MetI subunit is organized around a core of five transmembrane helices that correspond to a subset of the helices observed in the larger membrane-spanning subunits of the molybdate (ModBC) and maltose (MalFGK) ABC transporters. In addition to the conserved nucleotide binding domain of the ABC family, MetN contains a carboxyl-terminal extension with a ferredoxin-like fold previously assigned to a conserved family of regulatory ligand-binding domains. These domains separate the nucleotide binding domains and would interfere with their association required for ATP binding and hydrolysis. Methionine binds to the dimerized carboxyl-terminal domain and is shown to inhibit ATPase activity. These observations are consistent with an allosteric regulatory mechanism operating at the level of transport activity, where increased intracellular levels of the transported ligand stabilize an inward-facing, ATPase-inactive state of MetNI to inhibit further ligand translocation into the cell.
Figures
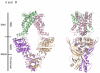
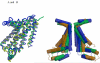
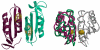
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

