Engineering ascorbate peroxidase activity into cytochrome c peroxidase
- PMID: 18771292
- PMCID: PMC2770236
- DOI: 10.1021/bi8007565
Engineering ascorbate peroxidase activity into cytochrome c peroxidase
Abstract
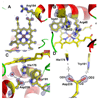
Cytochrome c peroxidase (CCP) and ascorbate peroxidase (APX) have very similar structures, and yet neither CCP nor APX exhibits each other's activities with respect to reducing substrates. APX has a unique substrate binding site near the heme propionates where ascorbate H-bonds with a surface Arg and one heme propionate (Sharp et al. (2003) Nat. Struct. Biol. 10, 303-307). The corresponding region in CCP has a much longer surface loop, and the critical Arg residue that is required for ascorbate binding in APX is Asn in CCP. In order to convert CCP into an APX, the ascorbate-binding loop and critical arginine were engineered into CCP to give the CCP2APX mutant. The mutant crystal structure shows that the engineered site is nearly identical to that found in APX. While wild-type CCP shows no APX activity, CCP2APX catalyzes the peroxidation of ascorbate at a rate of approximately 12 min (-1), indicating that the engineered ascorbate-binding loop can bind ascorbate.
Figures

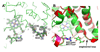
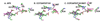

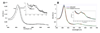

References
-
- Sivaraja M, Goodin DB, Smith M, Hoffman BM. Identification by ENDOR of Trp 191 as the free-radical site in cytochrome c peroxidase compound ES. Science. 1989;245:738–740. - PubMed
-
- Dunford HB. In: Horseradish peroxidase: structure and kinetic properties. Everse J, Everse KE, Grisham MB, editors. Boca Raton: CRC Press; 1991. pp. 1–24.
-
- Henriksen A, Smith AT, Gajhede MY. The structures of the horseradish peroxidase C-ferulic acid complex and the ternary complex with cyanide suggest how peroxidases oxidize small phenolic substrates. J. Biol. Chem. 1999;274:35005–35011. - PubMed
-
- Pelletier H, Kraut J. Crystal structure of a complex between electron transfer partners, cytochrome c peroxidase and cytochrome c. Science. 1992;258:1748–1755. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

