Structure of the redox sensor domain of Methylococcus capsulatus (Bath) MmoS
- PMID: 19271777
- PMCID: PMC2707821
- DOI: 10.1021/bi8019614
Structure of the redox sensor domain of Methylococcus capsulatus (Bath) MmoS
Abstract
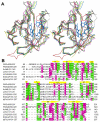
MmoS from Methylococcus capsulatus (Bath) is the multidomain sensor protein of a two-component signaling system proposed to play a role in the copper-mediated regulation of soluble methane monooxygenase (sMMO). MmoS binds an FAD cofactor within its N-terminal tandem Per-Arnt-Sim (PAS) domains, suggesting that it functions as a redox sensor. The crystal structure of the MmoS tandem PAS domains, designated PAS-A and PAS-B, has been determined to 2.34 A resolution. Both domains adopt the typical PAS domain alpha/beta topology and are structurally similar. The two domains are linked by a long alpha helix and do not interact with one another. The FAD cofactor is housed solely within PAS-A and is stabilized by an extended hydrogen bonding network. The overall fold of PAS-A is similar to those of other flavin-containing PAS domains, but homodimeric interactions in other structures are not observed in the MmoS sensor, which crystallized as a monomer. The structure both provides new insight into the architecture of tandem PAS domains and suggests specific residues that may play a role in MmoS FAD redox chemistry and subsequent signal transduction.
Figures



References
-
- Hefti M, Françoijs K-J, de Vries LC, Dixon R, Vervoort J. The PAS fold: a redefinition of the PAS domain based upon structural prediction. Eur. J. Biochem. 2004;271:1198–1208. - PubMed
-
- Ponting CP, Aravind L. PAS: a multifunctional domain family comes to light. Current Biol. 1997;7:R674–R677. - PubMed
-
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Ann. Rev. Biochem. 2000;69:183–215. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

