Active-site architecture and catalytic mechanism of the lipid A deacylase LpxR of Salmonella typhimurium
- PMID: 19174515
- PMCID: PMC2644146
- DOI: 10.1073/pnas.0813064106
Active-site architecture and catalytic mechanism of the lipid A deacylase LpxR of Salmonella typhimurium
Abstract
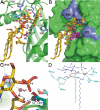
The lipid A portion of lipopolysaccharide, the major component of the outer leaflet of the outer membrane of gram-negative bacteria, is toxic to humans. Modification of lipid A by enzymes often reduces its toxicity. The outer-membrane protein LpxR from Salmonella typhimurium is a lipid A-modifying enzyme. It removes the 3'-acyloxyacyl moiety of the lipid A portion of lipopolysaccharide in a Ca(2+)-dependent manner. Here, we present the crystal structure of S. typhimurium LpxR, crystallized in the presence of zinc ions. The structure, a 12-stranded beta-barrel, reveals that the active site is located between the barrel wall and an alpha-helix formed by an extracellular loop. Based on site-directed mutagenesis and modeling of a substrate on the active site, we propose a catalytic mechanism similar to that of phospholipase A2, in which a Ca(2+) forms the oxyanion hole and a histidine activates a water molecule (or a cascade of two water molecules) that subsequently attacks the carbonyl oxygen of the scissile bond.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

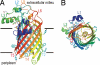
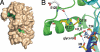
References
-
- Trent MS, Stead CM, Tran AX, Hankins JV. Diversity of endotoxin and its impact on pathogenesis. J Endotoxin Res. 2006;12:205–223. - PubMed
-
- Guo L, et al. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95:189–198. - PubMed
-
- Trent MS, Pabich W, Raetz CR, Miller SI. A PhoP/PhoQ-induced Lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J Biol Chem. 2001;276:9083–9092. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

