Crystal structure of Baeyer-Villiger monooxygenase MtmOIV, the key enzyme of the mithramycin biosynthetic pathway
- PMID: 19364090
- PMCID: PMC2713373
- DOI: 10.1021/bi8023509
Crystal structure of Baeyer-Villiger monooxygenase MtmOIV, the key enzyme of the mithramycin biosynthetic pathway
Abstract
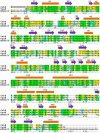
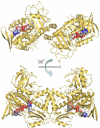
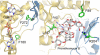
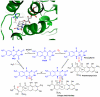
Baeyer-Villiger monooxygenases (BVMOs), mostly flavoproteins, were shown to be powerful biocatalysts for synthetic organic chemistry applications and were also suggested to play key roles for the biosyntheses of various natural products. Here we present the three-dimensional structure of MtmOIV, a 56 kDa homodimeric FAD- and NADPH-dependent monooxygenase, which catalyzes the key frame-modifying step of the mithramycin biosynthetic pathway and currently the only BVMO proven to react with its natural substrate via a Baeyer-Villiger reaction. MtmOIV's structure was determined by X-ray crystallography using molecular replacement to a resolution of 2.9 A. MtmOIV cleaves a C-C bond, essential for the conversion of the biologically inactive precursor, premithramycin B, into the active drug mithramycin. The MtmOIV structure combined with substrate docking calculations and site-directed mutagenesis experiments identifies several residues that participate in cofactor and substrate binding. Future experimentation aimed at broadening the substrate specificity of the enzyme could facilitate the generation of chemically diverse mithramycin analogues through combinatorial biosynthesis.
Figures



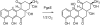

References
-
- Mihovilovic MD, Rudroff F, Grotzl B, Kapitan P, Snajdrova R, Rydz J, Mach R. Family clustering of Baeyer-Villiger monooxygenases based on protein sequence and stereopreference. Angew. Chem. Int. Ed. Engl. 2005;44:3609–3613. - PubMed
-
- van Berkel WJH, Kamerbeek NM, Fraaije MW. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 2006;124:670–689. - PubMed
-
- Kamerbeek NM, Fraaije MW, Janssen DB. Identifying determinants of NADPH specificity in Baeyer-Villiger monooxygenases. Eur. J. Biochem. 2004;271:2107–2116. - PubMed
-
- Kamerbeek NM, Janssen DB, van Berkel WJH, Fraaije MW. Baeyer-Villiger Monooxygenases, and Emerging Family of Flavin-Dependent Biocatalysts. Adv. Synth. Catal. 2003;345:667–678.
-
- Willetts A. Structural studies and synthetic applications of Baeyer-Villiger monooxygenases. Trends Biotechnol. 1997;15:55–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

