Selenomethionine incorporation in proteins expressed in Lactococcus lactis
- PMID: 19388077
- PMCID: PMC2771314
- DOI: 10.1002/pro.97
Selenomethionine incorporation in proteins expressed in Lactococcus lactis
Abstract
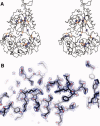
Lactococcus lactis is a promising host for (membrane) protein overproduction. Here, we describe a protocol for incorporation of selenomethionine (SeMet) into proteins expressed in L. lactis. Incorporation efficiencies of SeMet in the membrane protein complex OpuA (an ABC transporter) and the soluble protein OppA, both from L. lactis, were monitored by mass spectrometry. Both proteins incorporated SeMet with high efficiencies (>90%), which greatly extends the usefulness of the expression host L. lactis for X-ray crystallography purposes. The crystal structure of ligand-free OppA was determined at 2.4 A resolution by a semiautomatic approach using selenium single-wavelength anomalous diffraction phasing.
Figures

References
-
- Geertsma ER, Poolman B. High-throughput cloning and expression in recalcitrant bacteria. Nat Methods. 2007;4:705–707. - PubMed
-
- Morello E, Bermúdez-Humarán LG, Llull D, Solé V, Miraglio N, Langella P, Poquet I. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J Mol Microbiol Biotechnol. 2008;14:48–58. - PubMed
-
- Kunji ERS, Slotboom DJ, Poolman B. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim Biophys Acta. 2003;1610:97–108. - PubMed
-
- Kunji ERS, Chan KW, Slotboom DJ, Floyd S, O'Connor R, Monné M. Eukaryotic membrane protein overproduction in Lactococcus lactis. Curr Opin Biotechnol. 2005;16:546–551. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

