Switching catalysis from hydrolysis to perhydrolysis in Pseudomonas fluorescens esterase
- PMID: 20112920
- PMCID: PMC2855817
- DOI: 10.1021/bi9021268
Switching catalysis from hydrolysis to perhydrolysis in Pseudomonas fluorescens esterase
Abstract
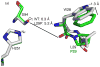
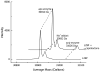
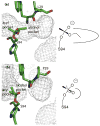
Many serine hydrolases catalyze perhydrolysis, the reversible formation of peracids from carboxylic acids and hydrogen peroxide. Recently, we showed that a single amino acid substitution in the alcohol binding pocket, L29P, in Pseudomonas fluorescens (SIK WI) aryl esterase (PFE) increased the specificity constant of PFE for peracetic acid formation >100-fold [Bernhardt et al. (2005) Angew. Chem., Int. Ed. 44, 2742]. In this paper, we extend this work to address the three following questions. First, what is the molecular basis of the increase in perhydrolysis activity? We previously proposed that the L29P substitution creates a hydrogen bond between the enzyme and hydrogen peroxide in the transition state. Here we report two X-ray structures of L29P PFE that support this proposal. Both structures show a main chain carbonyl oxygen closer to the active site serine as expected. One structure further shows acetate in the active site in an orientation consistent with reaction by an acyl-enzyme mechanism. We also detected an acyl-enzyme intermediate in the hydrolysis of epsilon-caprolactone by mass spectrometry. Second, can we further increase perhydrolysis activity? We discovered that the reverse reaction, hydrolysis of peracetic acid to acetic acid and hydrogen peroxide, occurs at nearly the diffusion limited rate. Since the reverse reaction cannot increase further, neither can the forward reaction. Consistent with this prediction, two variants with additional amino acid substitutions showed 2-fold higher k(cat), but K(m) also increased so the specificity constant, k(cat)/K(m), remained similar. Third, how does the L29P substitution change the esterase activity? Ester hydrolysis decreased for most esters (75-fold for ethyl acetate) but not for methyl esters. In contrast, L29P PFE catalyzed hydrolysis of epsilon-caprolactone five times more efficiently than wild-type PFE. Molecular modeling suggests that moving the carbonyl group closer to the active site blocks access for larger alcohol moieties but binds epsilon-caprolactone more tightly. These results are consistent with the natural function of perhydrolases being either hydrolysis of peroxycarboxylic acids or hydrolysis of lactones.
Figures



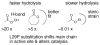
References
-
- Björkling F, Frykman H, Godtfredsen SE, Kirk O. Lipase-catalyzed synthesis of peroxycarboxylic acids and lipase-mediated oxidations. Tetrahedron. 1992;48:4587–4592.
-
- Picard M, Gross J, Lübbert E, Tölzer S, Krauss S, van Pée KH, Berkessel A. Metal-free bacterial haloperoxidases as unusual hydrolases: activation of H2O2 by the formation of peracetic acid. Angew Chem Int Ed Engl. 1997;36:1196–1199.
-
- Warwel S, Klaas MR. Chemoenzymic epoxidation of unsaturated carboxylic acids. J Mol Catal B: Enzym. 1995;1:29–35.
- Klaas MR, Warwel S. Lipase-catalyzed preparation of peroxy acids and their use for epoxidation. J Mol Catal A: Chem. 1997;117:311–319.
- de Zoete MC, van Rantwijk F, Maat L, Sheldon RA. Selective oxidation of penicillin G with hydrogen peroxide and with enzymatically generated peroxyoctanoic acid. Recl Trav Chim Pays-Bas. 1993;112:462–463.
-
- Hegarty AF. Comprehensive Organic Chemistry. Pearson Higher Education; New York: 1995. pp. 1105–1118.
-
-
Selected references: ten Brink GJ, Arends IWCE, Sheldon RA. The Baeyer-Villiger reaction: new developments toward greener procedures. Chem Rev. 2004;104:4105–4123.Harrison CR, Hodge P. Oxidation of some penicillins and other sulphides by use of a polymer-supported peroxy-acid. J Chem Soc Perkin Trans 1. 1976;21:2252–2254.Drago RS, Mateus ALML, Patton D. Stoichiometric and catalytic oxidation of organic substrates with in-situ-generated peracids. J Org Chem. 1996;61:5693–5696.Doumaux AR, McKeon ARJE, Trecker DJ. Metal ion-catalyzed peroxide oxidation of organic substrates Selective synthesis of imides. J Am Chem Soc. 1969;91:3992–3993.Sugimoto H, Sawyer DT. Iron(II)-induced activation of hydroperoxides for the dehydrogenation and monooxygenation of organic substrates in acetonitrile. J Am Chem Soc. 1985;107:5712–5716.
-
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

