Developing dual and specific inhibitors of dimethylarginine dimethylaminohydrolase-1 and nitric oxide synthase: toward a targeted polypharmacology to control nitric oxide
- PMID: 19663506
- PMCID: PMC2746464
- DOI: 10.1021/bi9007098
Developing dual and specific inhibitors of dimethylarginine dimethylaminohydrolase-1 and nitric oxide synthase: toward a targeted polypharmacology to control nitric oxide
Abstract
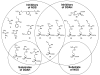
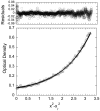
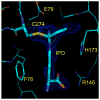
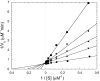
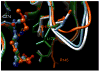
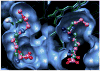
Molecules that block nitric oxide's (NO) biosynthesis are of significant interest. For example, nitric oxide synthase (NOS) inhibitors have been suggested as antitumor therapeutics, as have inhibitors of dimethylarginine dimethylaminohydrolase (DDAH), an enzyme that catabolizes endogenous NOS inhibitors. Dual-targeted inhibitors hold promise as more effective reagents to block NO biosynthesis than single-targeted compounds. In this study, a small set of known NOS inhibitors are surveyed as inhibitors of recombinant human DDAH-1. From these, an alkylamidine scaffold is selected for homologation. Stepwise lengthening of one substituent converts an NOS-selective inhibitor into a dual-targeted NOS/DDAH-1 inhibitor and then into a DDAH-1 selective inhibitor, as seen in the inhibition constants of N5-(1-iminoethyl)-, N5-(1-iminopropyl)-, N5-(1-iminopentyl)- and N(5)-(1-iminohexyl)-l-ornithine for neuronal NOS (1.7, 3, 20, >1,900 microM, respectively) and DDAH-1 (990, 52, 7.5, 110 microM, respectively). A 1.9 A X-ray crystal structure of the N5-(1-iminopropyl)-L-ornithine:DDAH-1 complex indicates covalent bond formation between the inhibitor's amidino carbon and the active-site Cys274, and solution studies show reversible competitive inhibition, consistent with a reversible covalent mode of DDAH inhibition by alkylamidine inhibitors. These represent a versatile scaffold for the development of a targeted polypharmacological approach to control NO biosynthesis.
Figures

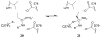
Similar articles
-
Developing an irreversible inhibitor of human DDAH-1, an enzyme upregulated in melanoma.ChemMedChem. 2014 Apr;9(4):792-7. doi: 10.1002/cmdc.201300557. Epub 2014 Feb 26. ChemMedChem. 2014. PMID: 24574257 Free PMC article.
-
S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase.Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13527-32. doi: 10.1073/pnas.212269799. Epub 2002 Oct 7. Proc Natl Acad Sci U S A. 2002. PMID: 12370443 Free PMC article.
-
Gene transfer of dimethylarginine dimethylaminohydrolase-2 improves the impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells induced by lysophosphatidylcholine.Eur J Pharmacol. 2008 Apr 14;584(1):49-56. doi: 10.1016/j.ejphar.2008.01.029. Epub 2008 Feb 5. Eur J Pharmacol. 2008. PMID: 18342305
-
Inhibitors of the Hydrolytic Enzyme Dimethylarginine Dimethylaminohydrolase (DDAH): Discovery, Synthesis and Development.Molecules. 2016 May 11;21(5):615. doi: 10.3390/molecules21050615. Molecules. 2016. PMID: 27187323 Free PMC article. Review.
-
The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis.J Pathol. 2013 Jan;229(2):242-9. doi: 10.1002/path.4127. J Pathol. 2013. PMID: 23097221 Review.
Cited by
-
Site-specific and regiospecific installation of methylarginine analogues into recombinant histones and insights into effector protein binding.J Am Chem Soc. 2013 Feb 27;135(8):2879-82. doi: 10.1021/ja3108214. Epub 2013 Feb 19. J Am Chem Soc. 2013. PMID: 23398247 Free PMC article.
-
Developing an irreversible inhibitor of human DDAH-1, an enzyme upregulated in melanoma.ChemMedChem. 2014 Apr;9(4):792-7. doi: 10.1002/cmdc.201300557. Epub 2014 Feb 26. ChemMedChem. 2014. PMID: 24574257 Free PMC article.
-
Selective Covalent Protein Modification by 4-Halopyridines through Catalysis.Chembiochem. 2017 Aug 4;18(15):1551-1556. doi: 10.1002/cbic.201700104. Epub 2017 Jun 27. Chembiochem. 2017. PMID: 28470883 Free PMC article.
-
Characterization of C-alkyl amidines as bioavailable covalent reversible inhibitors of human DDAH-1.ChemMedChem. 2011 Jan 3;6(1):81-8. doi: 10.1002/cmdc.201000392. ChemMedChem. 2011. PMID: 20979083 Free PMC article.
-
Ligand-dependent dynamics of the active-site lid in bacterial dimethylarginine dimethylaminohydrolase.Biochemistry. 2014 Feb 18;53(6):1092-104. doi: 10.1021/bi4015924. Epub 2014 Feb 7. Biochemistry. 2014. PMID: 24484052 Free PMC article.
References
-
- Hong L, Fast W. Inhibition of human dimethylargininase-1 (DDAH-1) by S-nitroso-L-homocysteine and hydrogen peroxide: analysis, quantification, and implications for hyperhomocysteinemia. J Biol Chem. 2007;282:34684–34692. - PubMed
-
- Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys. 2003;417:3–11. - PubMed
-
- Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311–20. - PubMed
-
- Wink DA, Mitchell JB. Nitric oxide and cancer: an introduction. Free Radic Biol Med. 2003;34:951–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

