Nonhomologous DNA end-joining for repair of DNA double-strand breaks
- PMID: 29247009
- PMCID: PMC6036208
- DOI: 10.1074/jbc.TM117.000374
Nonhomologous DNA end-joining for repair of DNA double-strand breaks
Abstract
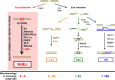
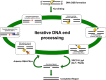
Nonhomologous DNA end-joining (NHEJ) is the predominant double-strand break (DSB) repair pathway throughout the cell cycle and accounts for nearly all DSB repair outside of the S and G2 phases. NHEJ relies on Ku to thread onto DNA termini and thereby improve the affinity of the NHEJ enzymatic components consisting of polymerases (Pol μ and Pol λ), a nuclease (the Artemis·DNA-PKcs complex), and a ligase (XLF·XRCC4·Lig4 complex). Each of the enzymatic components is distinctive for its versatility in acting on diverse incompatible DNA end configurations coupled with a flexibility in loading order, resulting in many possible junctional outcomes from one DSB. DNA ends can either be directly ligated or, if the ends are incompatible, processed until a ligatable configuration is achieved that is often stabilized by up to 4 bp of terminal microhomology. Processing of DNA ends results in nucleotide loss or addition, explaining why DSBs repaired by NHEJ are rarely restored to their original DNA sequence. Thus, NHEJ is a single pathway with multiple enzymes at its disposal to repair DSBs, resulting in a diversity of repair outcomes.
Keywords: DNA endonuclease; DNA repair; DNA-dependent serine/threonine protein kinase (DNA-PK); NHEJ; double-stranded DNA breaks; nucleic acid enzymology; protein structure.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

