Structural basis for substrate specificity of methylsuccinyl-CoA dehydrogenase, an unusual member of the acyl-CoA dehydrogenase family
- PMID: 29275330
- PMCID: PMC5798300
- DOI: 10.1074/jbc.RA117.000764
Structural basis for substrate specificity of methylsuccinyl-CoA dehydrogenase, an unusual member of the acyl-CoA dehydrogenase family
Abstract
(2S)-methylsuccinyl-CoA dehydrogenase (MCD) belongs to the family of FAD-dependent acyl-CoA dehydrogenase (ACD) and is a key enzyme of the ethylmalonyl-CoA pathway for acetate assimilation. It catalyzes the oxidation of (2S)-methylsuccinyl-CoA to α,β-unsaturated mesaconyl-CoA and shows only about 0.5% activity with succinyl-CoA. Here we report the crystal structure of MCD at a resolution of 1.37 Å. The enzyme forms a homodimer of two 60-kDa subunits. Compared with other ACDs, MCD contains an ∼170-residue-long N-terminal extension that structurally mimics a dimer-dimer interface of these enzymes that are canonically organized as tetramers. MCD catalyzes the unprecedented oxidation of an α-methyl branched dicarboxylic acid CoA thioester. Substrate specificity is achieved by a cluster of three arginines that accommodates the terminal carboxyl group and a dedicated cavity that facilitates binding of the C2 methyl branch. MCD apparently evolved toward preventing the nonspecific oxidation of succinyl-CoA, which is a close structural homolog of (2S)-methylsuccinyl-CoA and an essential intermediate in central carbon metabolism. For different metabolic engineering and biotechnological applications, however, an enzyme that can oxidize succinyl-CoA to fumaryl-CoA is sought after. Based on the MCD structure, we were able to shift substrate specificity of MCD toward succinyl-CoA through active-site mutagenesis.
Keywords: X-ray crystallography; acyl-CoA dehydrogenase; bioengineering; enzyme catalysis; enzyme kinetics; enzyme mutation; enzyme purification; enzyme structure; ethylmalonyl-CoA pathway; flavin adenine dinucleotide (FAD); glutaryl-CoA dehydrogenase; isobutyryl-CoA dehydrogenase; mesaconyl-CoA.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
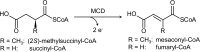


References
-
- Ikeda Y., Dabrowski C., and Tanaka K. (1983) Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria: identification of a new 2-methyl branched chain acyl-CoA dehydrogenase. J. Biol. Chem. 258, 1066–1076 - PubMed
-
- Izai K., Uchida Y., Orii T., Yamamoto S., and Hashimoto T. (1992) Novel fatty acid β-oxidation enzymes in rat liver mitochondria. I. Purification and properties of very-long-chain acyl-coenzyme A dehydrogenase. J. Biol. Chem. 267, 1027–1033 - PubMed
-
- Nguyen T. V., Andresen B. S., Corydon T. J., Ghisla S., Abd-El Razik N., Mohsen A. W., Cederbaum S. D., Roe D. S., Roe C. R., Lench N. J., and Vockley J. (2002) Identification of isobutyryl-CoA dehydrogenase and its deficiency in humans. Mol. Genet. Metab. 77, 68–79 10.1016/S1096-7192(02)00152-X - DOI - PubMed
-
- Besrat A., Polan C. E., and Henderson L. M. (1969) Mammalian metabolism of glutaric acid. J. Biol. Chem. 244, 1461–1467 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

