Role of zinc in isoform-selective inhibitor binding to neuronal nitric oxide synthase
- PMID: 21138269
- PMCID: PMC3193998
- DOI: 10.1021/bi1013479
Role of zinc in isoform-selective inhibitor binding to neuronal nitric oxide synthase
Abstract
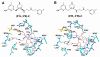
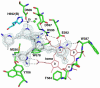
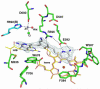
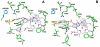
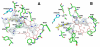
In previous studies [Delker, S. L., et al. (2010), J. Am. Chem. Soc. 132, 5437-5442], we determined the crystal structures of neuronal nitric oxide synthase (nNOS) in complex with nNOS-selective chiral pyrrolidine inhibitors, designed to have an aminopyridine group bound over the heme where it can electrostatically interact with the conserved active site Glu residue. However, in addition to the expected binding mode with the (S,S)-cis inhibitors, an unexpected "flipped" orientation was observed for the (R,R)-cis enantiomers. In the flipped mode, the aminopyridine extends out of the active site where it interacts with one heme propionate. This prompted us to design and synthesize symmetric "double-headed" inhibitors with an aminopyridine at each end of a bridging ring structure [Xue, F., Delker, S. L., Li, H., Fang, J., Jamal, J., Martásek, P., Roman, L. J., Poulos, T. L., and Silverman, R. B. Symmetric double-headed aminopyridines, a novel strategy for potent and membrane-permeable inhibitors of neuronal nitric oxide synthase. J. Med. Chem. (submitted for publication)]. One aminopyridine should interact with the active site Glu and the other with the heme propionate. Crystal structures of these double-headed aminopyridine inhibitors in complexes with nNOS show unexpected and significant protein and heme conformational changes induced by inhibitor binding that result in removal of the tetrahydrobiopterin (H(4)B) cofactor and creation of a new Zn(2+) site. These changes are due to binding of a second inhibitor molecule that results in the displacement of H(4)B and the placement of the inhibitor pyridine group in position to serve as a Zn(2+) ligand together with Asp, His, and a chloride ion. Binding of the second inhibitor molecule and generation of the Zn(2+) site do not occur in eNOS. Structural requirements for creation of the new Zn(2+) site in nNOS were analyzed in detail. These observations open the way for the potential design of novel inhibitors selective for nNOS.
Figures
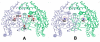

References
-
- Stuehr DJ, Griffith OW. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. - PubMed
-
- Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–374. - PubMed
-
- Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem. Int. 2002;40:511–526. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

