Folding, DNA recognition, and function of GIY-YIG endonucleases: crystal structures of R.Eco29kI
- PMID: 20800503
- PMCID: PMC2955809
- DOI: 10.1016/j.str.2010.07.006
Folding, DNA recognition, and function of GIY-YIG endonucleases: crystal structures of R.Eco29kI
Abstract
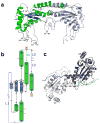
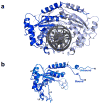
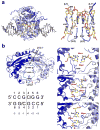
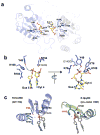
The GIY-YIG endonuclease family comprises hundreds of diverse proteins and a multitude of functions; none have been visualized bound to DNA. The structure of the GIY-YIG restriction endonuclease R.Eco29kI has been solved both alone and bound to its target site. The protein displays a domain-swapped homodimeric structure with several extended surface loops encircling the DNA. Only three side chains from each protein subunit contact DNA bases, two directly and one via a bridging solvent molecule. Both tyrosine residues within the GIY-YIG motif are positioned in the catalytic center near a putative nucleophilic water; the remainder of the active site resembles the HNH endonuclease family. The structure illustrates how the GIY-YIG scaffold has been adapted for the highly specific recognition of a DNA restriction site, in contrast to nonspecific DNA cleavage by GIY-YIG domains in homing endonucleases or structure-specific cleavage by DNA repair enzymes such as UvrC.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

