Structure of the putative dihydroorotate dehydrogenase from Streptococcus mutans
- PMID: 21301083
- PMCID: PMC3034605
- DOI: 10.1107/S1744309110048414
Structure of the putative dihydroorotate dehydrogenase from Streptococcus mutans
Abstract
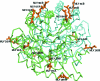
Streptococcus mutans is one of the pathogenic species involved in dental caries, especially in the initiation and development stages. Here, the crystal structure of SMU.595, a putative dihydroorotate dehydrogenase (DHOD) from S. mutans, is reported at 2.4 Å resolution. DHOD is a flavin mononucleotide-containing enzyme which catalyzes the oxidation of L-dihydroorotate to orotate, which is the fourth step and the only redox reaction in the de novo biosynthesis of pyrimidine nucleotides. The reductive lysine-methylation procedure was applied in order to improve the diffraction qualities of the crystals. Analysis of the S. mutans DHOD crystal structure shows that this enzyme is a class 1A DHOD and also suggests potential sites that could be exploited for the design of highly specific inhibitors using the structure-based chemotherapeutic design technique.
Figures



References
-
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

