Quantitative, directional measurement of electric field heterogeneity in the active site of ketosteroid isomerase
- PMID: 22308339
- PMCID: PMC3277571
- DOI: 10.1073/pnas.1111566109
Quantitative, directional measurement of electric field heterogeneity in the active site of ketosteroid isomerase
Abstract
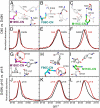
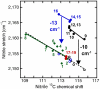
Understanding the electrostatic forces and features within highly heterogeneous, anisotropic, and chemically complex enzyme active sites and their connection to biological catalysis remains a longstanding challenge, in part due to the paucity of incisive experimental probes of electrostatic properties within proteins. To quantitatively assess the landscape of electrostatic fields at discrete locations and orientations within an enzyme active site, we have incorporated site-specific thiocyanate vibrational probes into multiple positions within bacterial ketosteroid isomerase. A battery of X-ray crystallographic, vibrational Stark spectroscopy, and NMR studies revealed electrostatic field heterogeneity of 8 MV/cm between active site probe locations and widely differing sensitivities of discrete probes to common electrostatic perturbations from mutation, ligand binding, and pH changes. Electrostatic calculations based on active site ionization states assigned by literature precedent and computational pK(a) prediction were unable to quantitatively account for the observed vibrational band shifts. However, electrostatic models of the D40N mutant gave qualitative agreement with the observed vibrational effects when an unusual ionization of an active site tyrosine with a pK(a) near 7 was included. UV-absorbance and (13)C NMR experiments confirmed the presence of a tyrosinate in the active site, in agreement with electrostatic models. This work provides the most direct measure of the heterogeneous and anisotropic nature of the electrostatic environment within an enzyme active site, and these measurements provide incisive benchmarks for further developing accurate computational models and a foundation for future tests of electrostatics in enzymatic catalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Selzer T, Schreiber G. Predicting the rate enhancement of protein complex formation from the electrostatic energy of interaction. J Mol Biol. 1999;287:409–419. - PubMed
-
- Lee L-P, Tidor B. Barstar is electrostatically optimized for tight binding to barnase. Nat Struct Mol Biol. 2001;8:73–76. - PubMed
-
- Getzoff ED, et al. Faster superoxide dismutase mutants designed by enhancing electrostatic guidance. Nature. 1992;358:347–351. - PubMed
-
- Dong F, Zhou H. Electrostatic contribution to the binding stability of protein-protein complexes. Proteins. 2006;65:87–102. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

