Atomic-level modelling of the HIV capsid
- PMID: 21248851
- PMCID: PMC3075868
- DOI: 10.1038/nature09640
Atomic-level modelling of the HIV capsid
Abstract
The mature capsids of human immunodeficiency virus type 1 (HIV-1) and other retroviruses are fullerene shells, composed of the viral CA protein, that enclose the viral genome and facilitate its delivery into new host cells. Retroviral CA proteins contain independently folded amino (N)- and carboxy (C)-terminal domains (NTD and CTD) that are connected by a flexible linker. The NTD forms either hexameric or pentameric rings, whereas the CTD forms symmetric homodimers that connect the rings into a hexagonal lattice. We previously used a disulphide crosslinking strategy to enable isolation and crystallization of soluble HIV-1 CA hexamers. Here we use the same approach to solve the X-ray structure of the HIV-1 CA pentamer at 2.5 Å resolution. Two mutant CA proteins with engineered disulphides at different positions (P17C/T19C and N21C/A22C) converged onto the same quaternary structure, indicating that the disulphide-crosslinked proteins recapitulate the structure of the native pentamer. Assembly of the quasi-equivalent hexamers and pentamers requires remarkably subtle rearrangements in subunit interactions, and appears to be controlled by an electrostatic switch that favours hexamers over pentamers. This study completes the gallery of substructures describing the components of the HIV-1 capsid and enables atomic-level modelling of the complete capsid. Rigid-body rotations around two assembly interfaces appear sufficient to generate the full range of continuously varying lattice curvature in the fullerene cone.
Figures

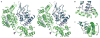
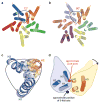
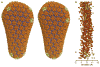
References
-
- Gitti RK, et al. Structure of the amino-terminal core domain of the HIV-1 capsid protein. Science. 1996;273:231–235. - PubMed
-
- Gamble TR, et al. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science. 1997;278:849–853. - PubMed
-
- Worthylake DK, Wang H, Yoo S, Sundquist WI, Hill CP. Structures of the HIV-1 capsid protein dimerization domain at 2.6 A resolution. Acta Crystallogr D Biol Crystallogr. 1999;55:85–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

