Small terminase couples viral DNA binding to genome-packaging ATPase activity
- PMID: 22771211
- PMCID: PMC3563279
- DOI: 10.1016/j.str.2012.05.014
Small terminase couples viral DNA binding to genome-packaging ATPase activity
Abstract
Packaging of viral genomes into empty procapsids is powered by a large DNA-packaging motor. In most viruses, this machine is composed of a large (L) and a small (S) terminase subunit complexed with a dodecamer of portal protein. Here we describe the 1.75 Å crystal structure of the bacteriophage P22 S-terminase in a nonameric conformation. The structure presents a central channel ∼23 Å in diameter, sufficiently large to accommodate hydrated B-DNA. The last 23 residues of S-terminase are essential for binding to DNA and assembly to L-terminase. Upon binding to its own DNA, S-terminase functions as a specific activator of L-terminase ATPase activity. The DNA-dependent stimulation of ATPase activity thus rationalizes the exclusive specificity of genome-packaging motors for viral DNA in the crowd of host DNA, ensuring fidelity of packaging and avoiding wasteful ATP hydrolysis. This posits a model for DNA-dependent activation of genome-packaging motors of general interest in virology.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


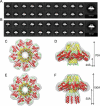

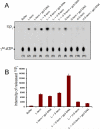
Comment in
-
Themes and variations of viral small terminase proteins.Structure. 2012 Aug 8;20(8):1291-2. doi: 10.1016/j.str.2012.07.007. Structure. 2012. PMID: 22884105 Free PMC article.
References
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42.
-
- Ackermann HW. Bacteriophage observations and evolution. Res Microbiol. 2003;154:245–251. - PubMed
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–1954. - PubMed
-
- Baumann RG, Black LW. Isolation and characterization of T4 bacteriophage gp17 terminase, a large subunit multimer with enhanced ATPase activity. J Biol Chem. 2003;278:4618–4627. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

