Structural and biochemical characterization of 20β-hydroxysteroid dehydrogenase from Bifidobacterium adolescentis strain L2-32
- PMID: 31209107
- PMCID: PMC6690682
- DOI: 10.1074/jbc.RA119.009390
Structural and biochemical characterization of 20β-hydroxysteroid dehydrogenase from Bifidobacterium adolescentis strain L2-32
Abstract
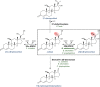
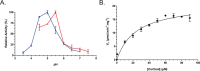
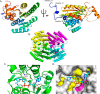
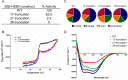
Anaerobic bacteria inhabiting the human gastrointestinal tract have evolved various enzymes that modify host-derived steroids. The bacterial steroid-17,20-desmolase pathway cleaves the cortisol side chain, forming pro-androgens predicted to impact host physiology. Bacterial 20β-hydroxysteroid dehydrogenase (20β-HSDH) regulates cortisol side-chain cleavage by reducing the C-20 carboxyl group on cortisol, yielding 20β-dihydrocortisol. Recently, the gene encoding 20β-HSDH in Butyricicoccus desmolans ATCC 43058 was reported, and a nonredundant protein search yielded a candidate 20β-HSDH gene in Bifidobacterium adolescentis strain L2-32. B. adolescentis 20β-HSDH could regulate cortisol side-chain cleavage by limiting pro-androgen formation in bacteria such as Clostridium scindens and 21-dehydroxylation by Eggerthella lenta Here, the putative B. adolescentis 20β-HSDH was cloned, overexpressed, and purified. 20β-HSDH activity was confirmed through whole-cell and pure enzymatic assays, and it is specific for cortisol. Next, we solved the structures of recombinant 20β-HSDH in both the apo- and holo-forms at 2.0-2.2 Å resolutions, revealing close overlap except for rearrangements near the active site. Interestingly, the structures contain a large, flexible N-terminal region that was investigated by gel-filtration chromatography and CD spectroscopy. This extended N terminus is important for protein stability because deletions of varying lengths caused structural changes and reduced enzymatic activity. A nonconserved extended N terminus was also observed in several short-chain dehydrogenase/reductase family members. B. adolescentis strains capable of 20β-HSDH activity could alter glucocorticoid metabolism in the gut and thereby serve as potential probiotics for the management of androgen-dependent diseases.
Keywords: 20β-hydroxysteroid dehydrogenase; Bifidobacteria; C-20 reduction; Steroid-17,20-desmolase; androgen; cortisol; glucocorticoid; microbiome; oxidation–reduction (redox); probiotic.
© 2019 Doden et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Ridlon J. M., Ikegawa S., Alves J. M., Zhou B., Kobayashi A., Iida T., Mitamura K., Tanabe G., Serrano M., De Guzman A., Cooper P., Buck G. A., and Hylemon P. B. (2013) Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 54, 2437–2449 10.1194/jlr.M038869 - DOI - PMC - PubMed
-
- Mythen S. M., Devendran S., Méndez-García C., Cann I., and Ridlon J. M. (2018) Targeted synthesis and characterization of a gene cluster encoding NAD(P)H-dependent 3α-, 3β-, and 12α-hydroxysteroid dehydrogenases from Eggerthella CAG:298, a gut metagenomic sequence. Appl. Environ. Microbiol. 84, e02475–17 10.1128/AEM.02475-17 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

