Principles of activation and permeation in an anion-selective Cys-loop receptor
- PMID: 21572436
- PMCID: PMC3160419
- DOI: 10.1038/nature10139
Principles of activation and permeation in an anion-selective Cys-loop receptor
Abstract
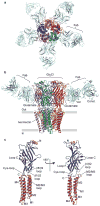
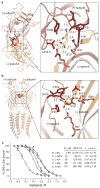
Fast inhibitory neurotransmission is essential for nervous system function and is mediated by binding of inhibitory neurotransmitters to receptors of the Cys-loop family embedded in the membranes of neurons. Neurotransmitter binding triggers a conformational change in the receptor, opening an intrinsic chloride channel and thereby dampening neuronal excitability. Here we present the first three-dimensional structure, to our knowledge, of an inhibitory anion-selective Cys-loop receptor, the homopentameric Caenorhabditis elegans glutamate-gated chloride channel α (GluCl), at 3.3 Å resolution. The X-ray structure of the GluCl-Fab complex was determined with the allosteric agonist ivermectin and in additional structures with the endogenous neurotransmitter L-glutamate and the open-channel blocker picrotoxin. Ivermectin, used to treat river blindness, binds in the transmembrane domain of the receptor and stabilizes an open-pore conformation. Glutamate binds in the classical agonist site at subunit interfaces, and picrotoxin directly occludes the pore near its cytosolic base. GluCl provides a framework for understanding mechanisms of fast inhibitory neurotransmission and allosteric modulation of Cys-loop receptors.
Figures


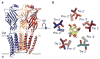

References
-
- Hille B. Ion Channels of Excitable Membranes. Sinauer Associates, Inc.; Sunderland, MA: 2001.
-
- Thompson AJ, Lester HA, Lummis SC. The structural basis of function in Cys-loop receptors. Q Rev Biophys. 2010;43:449–499. - PubMed
-
- Hilf RJ, Dutzler R. A prokaryotic perspective on pentameric ligand-gated ion channel structure. Curr Opin Struct Biol. 2009;19:418–424. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

