Human noroviruses' fondness for histo-blood group antigens
- PMID: 25428879
- PMCID: PMC4338890
- DOI: 10.1128/JVI.02968-14
Human noroviruses' fondness for histo-blood group antigens
Abstract
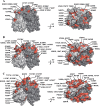
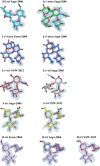
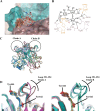
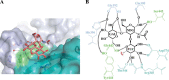
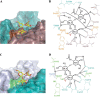
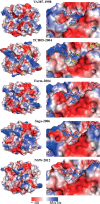
Human noroviruses are the dominant cause of outbreaks of gastroenteritis around the world. Human noroviruses interact with the polymorphic human histo-blood group antigens (HBGAs), and this interaction is thought to be important for infection. Indeed, synthetic HBGAs or HBGA-expressing enteric bacteria were shown to enhance norovirus infection in B cells. A number of studies have found a possible relationship between HBGA type and norovirus susceptibility. The genogroup II, genotype 4 (GII.4) noroviruses are the dominant cluster, evolve every other year, and are thought to modify their binding interactions with different HBGA types. Here we show high-resolution X-ray crystal structures of the capsid protruding (P) domains from epidemic GII.4 variants from 2004, 2006, and 2012, cocrystallized with a panel of HBGA types (H type 2, Lewis Y, Lewis B, Lewis A, Lewis X, A type, and B type). Many of the HBGA binding interactions were found to be complex, involving capsid loop movements, alternative HBGA conformations, and HBGA rotations. We showed that a loop (residues 391 to 395) was elegantly repositioned to allow for Lewis Y binding. This loop was also slightly shifted to provide direct hydrogen- and water-mediated bonds with Lewis B. We considered that the flexible loop modulated Lewis HBGA binding. The GII.4 noroviruses have dominated outbreaks over the past decade, which may be explained by their exquisite HBGA binding mechanisms, their fondness for Lewis HBGAs, and their temporal amino acid modifications.
Importance: Our data provide a comprehensive picture of GII.4 P domain and HBGA binding interactions. The exceptionally high resolutions of our X-ray crystal structures allowed us to accurately recognize novel GII.4 P domain interactions with numerous HBGA types. We showed that the GII.4 P domain-HBGA interactions involved complex binding mechanisms that were not previously observed in norovirus structural studies. Many of the GII.4 P domain-HBGA interactions we identified were negative in earlier enzyme-linked immunosorbent assay (ELISA)-based studies. Altogether, our data show that the GII.4 norovirus P domains can accommodate numerous HBGA types.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






Similar articles
-
Structural analysis of a rabbit hemorrhagic disease virus binding to histo-blood group antigens.J Virol. 2015 Feb;89(4):2378-87. doi: 10.1128/JVI.02832-14. Epub 2014 Dec 10. J Virol. 2015. PMID: 25505081 Free PMC article.
-
Crystal structures of GII.10 and GII.12 norovirus protruding domains in complex with histo-blood group antigens reveal details for a potential site of vulnerability.J Virol. 2011 Jul;85(13):6687-701. doi: 10.1128/JVI.00246-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525337 Free PMC article.
-
Epitope mapping of histo blood group antigens bound to norovirus VLPs using STD NMR experiments reveals fine details of molecular recognition.Glycoconj J. 2017 Oct;34(5):679-689. doi: 10.1007/s10719-017-9792-5. Epub 2017 Aug 19. Glycoconj J. 2017. PMID: 28823097
-
Human Norovirus Interactions with Histo-Blood Group Antigens and Human Milk Oligosaccharides.J Virol. 2016 Jun 10;90(13):5855-5859. doi: 10.1128/JVI.00317-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27122582 Free PMC article. Review.
-
Norovirus drug candidates that inhibit viral capsid attachment to human histo-blood group antigens.Antiviral Res. 2016 Sep;133:14-22. doi: 10.1016/j.antiviral.2016.07.006. Epub 2016 Jul 13. Antiviral Res. 2016. PMID: 27421712 Free PMC article. Review.
Cited by
-
Pandemic GII.4 Sydney and Epidemic GII.17 Kawasaki308 Noroviruses Display Distinct Specificities for Histo-Blood Group Antigens Leading to Different Transmission Vector Dynamics in Pacific Oysters.Front Microbiol. 2018 Nov 27;9:2826. doi: 10.3389/fmicb.2018.02826. eCollection 2018. Front Microbiol. 2018. PMID: 30542329 Free PMC article.
-
The Double Face of Mucin-Type O-Glycans in Lectin-Mediated Infection and Immunity.Molecules. 2018 May 11;23(5):1151. doi: 10.3390/molecules23051151. Molecules. 2018. PMID: 29751628 Free PMC article. Review.
-
The Antigenic Topology of Norovirus as Defined by B and T Cell Epitope Mapping: Implications for Universal Vaccines and Therapeutics.Viruses. 2019 May 10;11(5):432. doi: 10.3390/v11050432. Viruses. 2019. PMID: 31083353 Free PMC article. Review.
-
Carbon Dots' Antiviral Functions Against Noroviruses.Sci Rep. 2017 Mar 31;7(1):519. doi: 10.1038/s41598-017-00675-x. Sci Rep. 2017. PMID: 28364126 Free PMC article.
-
Development of a broad-spectrum therapeutic Fc-nanobody for human noroviruses.J Virol. 2024 Jul 23;98(7):e0070724. doi: 10.1128/jvi.00707-24. Epub 2024 Jul 2. J Virol. 2024. PMID: 38953655 Free PMC article.
References
-
- Hansman GS, Natori K, Shirato-Horikoshi H, Ogawa S, Oka T, Katayama K, Tanaka T, Miyoshi T, Sakae K, Kobayashi S, Shinohara M, Uchida K, Sakurai N, Shinozaki K, Okada M, Seto Y, Kamata K, Nagata N, Tanaka K, Miyamura T, Takeda N. 2006. Genetic and antigenic diversity among noroviruses. J Gen Virol 87:909–919. doi:10.1099/vir.0.81532-0. - DOI - PubMed
-
- Debbink K, Lindesmith LC, Donaldson EF, Costantini V, Beltramello M, Corti D, Swanstrom J, Lanzavecchia A, Vinje J, Baric RS. 2013. Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J Infect Dis 208:1877–1887. doi:10.1093/infdis/jit370. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

