Structural basis for specificity of TGFβ family receptor small molecule inhibitors
- PMID: 21983015
- PMCID: PMC4490768
- DOI: 10.1016/j.cellsig.2011.09.027
Structural basis for specificity of TGFβ family receptor small molecule inhibitors
Abstract
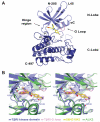
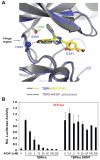
Transforming growth factor-β (TGFβ) receptor kinase inhibitors have a great therapeutic potential. SB431542 is one of the mainly used kinase inhibitors of the TGFβ/Activin pathway receptors, but needs improvement of its EC(50) (EC(50)=1 μM) to be translated to clinical use. A key feature of SB431542 is that it specifically targets receptors from the TGFβ/Activin pathway but not the closely related receptors from the bone morphogenic proteins (BMP) pathway. To understand the mechanisms of this selectivity, we solved the crystal structure of the TGFβ type I receptor (TβRI) kinase domain in complex with SB431542. We mutated TβRI residues coordinating SB431542 to their counterparts in activin-receptor like kinase 2 (ALK2), a BMP receptor kinase, and tested the kinase activity of mutated TβRI. We discovered that a Ser280Thr mutation yielded a TβRI variant that was resistant to SB431542 inhibition. Furthermore, the corresponding Thr283Ser mutation in ALK2 yielded a BMP receptor sensitive to SB431542. This demonstrated that Ser280 is the key determinant of selectivity for SB431542. This work provides a framework for optimising the SB431542 scaffold to more potent and selective inhibitors of the TGFβ/Activin pathway.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Attisano L, Wrana JL. Science. 2002;296:1646–1647. - PubMed
-
- Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R, Mathur A, Olson BA, Weinstock J, Laping NJ. Journal of medicinal chemistry. 2002;45:999–1001. - PubMed
-
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. Mol Pharmacol. 2002;62:65–74. - PubMed
-
- Fu K, Corbley MJ, Sun L, Friedman JE, Shan F, Papadatos JL, Costa D, Lutterodt F, Sweigard H, Bowes S, Choi M, Boriack-Sjodin PA, Arduini RM, Sun D, Newman MN, Zhang X, Mead JN, Chuaqui CE, Cheung HK, Cornebise M, Carter MB, Josiah S, Singh J, Lee WC, Gill A, Ling LE. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:665–671. - PubMed
-
- Singh J, Chuaqui CE, Boriack-Sjodin PA, Lee WC, Pontz T, Corbley MJ, Cheung HK, Arduini RM, Mead JN, Newman MN, Papadatos JL, Bowes S, Josiah S, Ling LE. Bioorganic & medicinal chemistry letters. 2003;13:4355–4359. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

