Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel
- PMID: 22282805
- PMCID: PMC3329120
- DOI: 10.1126/science.1213808
Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel
Abstract
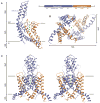
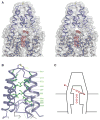
TRAAK channels, members of the two-pore domain K(+) (potassium ion) channel family K2P, are expressed almost exclusively in the nervous system and control the resting membrane potential. Their gating is sensitive to polyunsaturated fatty acids, mechanical deformation of the membrane, and temperature changes. Physiologically, these channels appear to control the noxious input threshold for temperature and pressure sensitivity in dorsal root ganglia neurons. We present the crystal structure of human TRAAK at a resolution of 3.8 angstroms. The channel comprises two protomers, each containing two distinct pore domains, which create a two-fold symmetric K(+) channel. The extracellular surface features a helical cap, 35 angstroms tall, that creates a bifurcated pore entryway and accounts for the insensitivity of two-pore domain K(+) channels to inhibitory toxins. Two diagonally opposed gate-forming inner helices form membrane-interacting structures that may underlie this channel's sensitivity to chemical and mechanical properties of the cell membrane.
Figures



Comment in
-
Structural biology. The inner workings of a dynamic duo.Science. 2012 Jan 27;335(6067):416-7. doi: 10.1126/science.1217679. Science. 2012. PMID: 22282800 No abstract available.
-
Two-pore domain potassium channels: variation on a structural theme.Channels (Austin). 2012 May-Jun;6(3):139-40. doi: 10.4161/chan.20973. Epub 2012 May 1. Channels (Austin). 2012. PMID: 22699405 Free PMC article.
References
-
- Hille B. Ion channels of excitable membranes. Sinauer Associates Inc; Sunderland, MA: 2001.
-
- Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 90:559. - PubMed
-
- Ketchum KA, Joiner WJ, Sellers AJ, Kaczmarek LK, Goldstein SA. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690. - PubMed
-
- Zhou XL, Vaillant B, Loukin SH, Kung C, Saimi Y. YKC1 encodes the depolarization-activated K+ channel in the plasma membrane of yeast. FEBS Lett. 1995;373:170. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

