Crystal structure and characterization of the glycoside hydrolase family 62 α-L-arabinofuranosidase from Streptomyces coelicolor
- PMID: 24482228
- PMCID: PMC3953306
- DOI: 10.1074/jbc.M113.540542
Crystal structure and characterization of the glycoside hydrolase family 62 α-L-arabinofuranosidase from Streptomyces coelicolor
Abstract
α-L-arabinofuranosidase, which belongs to the glycoside hydrolase family 62 (GH62), hydrolyzes arabinoxylan but not arabinan or arabinogalactan. The crystal structures of several α-L-arabinofuranosidases have been determined, although the structures, catalytic mechanisms, and substrate specificities of GH62 enzymes remain unclear. To evaluate the substrate specificity of a GH62 enzyme, we determined the crystal structure of α-L-arabinofuranosidase, which comprises a carbohydrate-binding module family 13 domain at its N terminus and a catalytic domain at its C terminus, from Streptomyces coelicolor. The catalytic domain was a five-bladed β-propeller consisting of five radially oriented anti-parallel β-sheets. Sugar complex structures with l-arabinose, xylotriose, and xylohexaose revealed five subsites in the catalytic cleft and an l-arabinose-binding pocket at the bottom of the cleft. The entire structure of this GH62 family enzyme was very similar to that of glycoside hydrolase 43 family enzymes, and the catalytically important acidic residues found in family 43 enzymes were conserved in GH62. Mutagenesis studies revealed that Asp(202) and Glu(361) were catalytic residues, and Trp(270), Tyr(461), and Asn(462) were involved in the substrate-binding site for discriminating the substrate structures. In particular, hydrogen bonding between Asn(462) and xylose at the nonreducing end subsite +2 was important for the higher activity of substituted arabinofuranosyl residues than that for terminal arabinofuranoses.
Keywords: Crystal Structure; Enzyme Kinetics; Enzyme Mechanisms; Enzyme Mutation; Enzyme Structure; Glycoside Hydrolase Family 62; Streptomyces coelicolor; Substrate Specificity; α -l-Arabinofuranosidase.
Figures
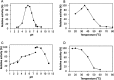
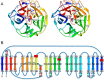
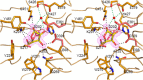

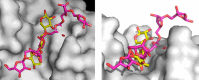

References
-
- Carvalheiro F., Duarte L. C., Girio F. M. (2008) Hemicellulose biorefineries: a review on biomass pretreatments. J. Sci. Indust. Res. 67, 849–864
-
- Pauly M., Keegstra K. (2008) Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 54, 559–568 - PubMed
-
- Pauly M., Gille S., Liu L., Mansoori N., de Souza A., Schultink A., Xiong G. (2013) Hemicellulose biosynthesis. Planta 238, 627–642 - PubMed
-
- Saha B. C. (2000) α-l-Arabinofuranosidase: biochemistry, molecular biology and application in biotechnology. Biotechnol Adv. 18, 403–423 - PubMed
-
- Kaneko S., Shimasaki T., Kusakabe I. (1993) Purification and some properties of intracellular α-l-arabinofuranosidase from Aspergillus niger 5-16. Biosci. Biotechnol. Biochem. 57, 1161–1165 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

