Subangstrom resolution X-ray structure details aquaporin-water interactions
- PMID: 23766328
- PMCID: PMC4066176
- DOI: 10.1126/science.1234306
Subangstrom resolution X-ray structure details aquaporin-water interactions
Abstract
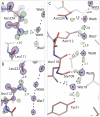
Aquaporins are membrane channels that facilitate the flow of water across biological membranes. Two conserved regions are central for selective function: the dual asparagine-proline-alanine (NPA) aquaporin signature motif and the aromatic and arginine selectivity filter (SF). Here, we present the crystal structure of a yeast aquaporin at 0.88 angstrom resolution. We visualize the H-bond donor interactions of the NPA motif's asparagine residues to passing water molecules; observe a polarized water-water H-bond configuration within the channel; assign the tautomeric states of the SF histidine and arginine residues; and observe four SF water positions too closely spaced to be simultaneously occupied. Strongly correlated movements break the connectivity of SF waters to other water molecules within the channel and prevent proton transport via a Grotthuss mechanism.
Figures



Comment in
-
Biochemistry. Watch water flow.Science. 2013 Jun 14;340(6138):1294-5. doi: 10.1126/science.1239270. Science. 2013. PMID: 23766318 No abstract available.
References
-
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687. - PubMed
-
- de Grotthuss CJT. Sur la décomposition de l’eau et des corps qu’elle tient en dissolution à l’aide de l’électricité galvanique. Ann. Chim. 1806;58:54.
-
- Marx D. Proton transfer 200 years after von Grotthuss: insights from ab initio simulations. Chemphyschem. 2006;7:1848. - PubMed
-
- Fu D, et al. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

