Structural basis for DNA recognition and loading into a viral packaging motor
- PMID: 22207627
- PMCID: PMC3271932
- DOI: 10.1073/pnas.1110270109
Structural basis for DNA recognition and loading into a viral packaging motor
Abstract
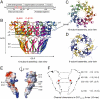
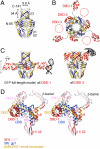
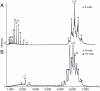
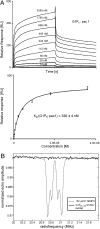
Genome packaging into preformed viral procapsids is driven by powerful molecular motors. The small terminase protein is essential for the initial recognition of viral DNA and regulates the motor's ATPase and nuclease activities during DNA translocation. The crystal structure of a full-length small terminase protein from the Siphoviridae bacteriophage SF6, comprising the N-terminal DNA binding, the oligomerization core, and the C-terminal β-barrel domains, reveals a nine-subunit circular assembly in which the DNA-binding domains are arranged around the oligomerization core in a highly flexible manner. Mass spectrometry analysis and four further crystal structures show that, although the full-length protein exclusively forms nine-subunit assemblies, protein constructs missing the C-terminal β-barrel form both nine-subunit and ten-subunit assemblies, indicating the importance of the C terminus for defining the oligomeric state. The mechanism by which a ring-shaped small terminase oligomer binds viral DNA has not previously been elucidated. Here, we probed binding in vitro by using EPR and surface plasmon resonance experiments, which indicated that interaction with DNA is mediated exclusively by the DNA-binding domains and suggested a nucleosome-like model in which DNA binds around the outside of the protein oligomer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
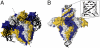
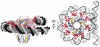
References
-
- Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu Rev Genet. 2008;42:647–681. - PubMed
-
- Casjens SR. The DNA-packaging nanomotor of tailed bacteriophages. Nat Rev Microbiol. 2011;9:647–657. - PubMed
-
- Earnshaw WC, Casjens SR. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980;21:319–331. - PubMed
-
- Smith DE, et al. The bacteriophage ϕ29 portal motor can package DNA against a large internal force. Nature. 2001;413:748–752. - PubMed
-
- Camacho AG, Gual A, Lurz R, Tavares P, Alonso JC. Bacillus subtilis bacteriophage SPP1 DNA packaging motor requires terminase and portal proteins. J Biol Chem. 2003;278:23251–23259. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

