Chromophorylation of cyanobacteriochrome Slr1393 from Synechocystis sp. PCC 6803 is regulated by protein Slr2111 through allosteric interaction
- PMID: 30242127
- PMCID: PMC6240869
- DOI: 10.1074/jbc.RA118.003830
Chromophorylation of cyanobacteriochrome Slr1393 from Synechocystis sp. PCC 6803 is regulated by protein Slr2111 through allosteric interaction
Abstract
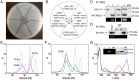
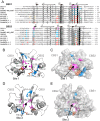
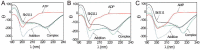
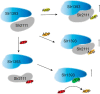
Cyanobacteriochromes (CBCRs) are photochromic proteins in cyanobacteria that act as photosensors. CBCRs bind bilins as chromophores and sense nearly the entire visible spectrum of light, but the regulation of the chromophorylation of CBCRs is unknown. Slr1393 from Synechocystis sp. PCC 6803 is a CBCR containing three consecutive GAF (cGMP phosphodiesterase, adenylyl cyclase, and FhlA protein) domains, of which only the third one (Slr1393g3) can be phycocyanobilin-chromophorylated. The protein Slr2111 from Synechocystis sp. PCC 6803 includes a cystathionine β-synthase (CBS) domain pair of an as yet unknown function at its N terminus. CBS domains are often characterized as sensors of cellular energy status by binding nucleotides. In this work, we demonstrate that Slr2111 strongly interacts with Slr1393 in vivo and in vitro, which generates a complex in a 1:1 molar ratio. This tight interaction inhibits the chromophorylation of Slr1393g3, even if the chromophore is present. Instead, the complex stability and thereby the chromophorylation of Slr1393 are regulated by the binding of nucleotides (ATP, ADP, AMP) to the CBS domains of Slr2111 with varying affinities. It is demonstrated that residues Asp-53 and Arg-97 of Slr2111 are involved in nucleotide binding. While ATP binds to Slr2111, the association between the two proteins gets weaker and chromophorylation of Slr1393 are enabled. In contrast, AMP binding to Slr2111 leads to a stronger association, thereby inhibiting the chromophorylation. It is concluded that Slr2111 acts as a sensor of the cellular energy status that regulates the chromophorylation of Slr1393 and thereby its function as a light-driven histidine kinase.
Keywords: adenosine-binding protein; co-immunoprecipitation; cyanobacteria; cystathionine beta-synthase domain; photoreceptor; photosynthesis; photosynthetic pigment; phototransduction; phycocyanobilin; protein-protein interaction; regulation.
© 2018 He et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Chen Y., Zhang J., Luo J., Tu J. M., Zeng X. L., Xie J., Zhou M., Zhao J. Q., Scheer H., and Zhao K. H. (2012) Photophysical diversity of two novel cyanobacteriochromes with phycocyanobilin chromophores: photochemistry and dark reversion kinetics. FEBS J. 279, 40–54 10.1111/j.1742-4658.2011.08397.x - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

