Structures of human constitutive nitric oxide synthases
- PMID: 25286850
- PMCID: PMC4188008
- DOI: 10.1107/S1399004714017064
Structures of human constitutive nitric oxide synthases
Abstract
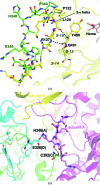
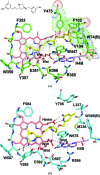
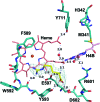
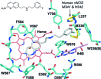
Mammals produce three isoforms of nitric oxide synthase (NOS): neuronal NOS (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS). The overproduction of NO by nNOS is associated with a number of neurodegenerative disorders; therefore, a desirable therapeutic goal is the design of drugs that target nNOS but not the other isoforms. Crystallography, coupled with computational approaches and medicinal chemistry, has played a critical role in developing highly selective nNOS inhibitors that exhibit exceptional neuroprotective properties. For historic reasons, crystallography has focused on rat nNOS and bovine eNOS because these were available in high quality; thus, their structures have been used in structure-activity-relationship studies. Although these constitutive NOSs share more than 90% sequence identity across mammalian species for each NOS isoform, inhibitor-binding studies revealed that subtle differences near the heme active site in the same NOS isoform across species still impact enzyme-inhibitor interactions. Therefore, structures of the human constitutive NOSs are indispensible. Here, the first structure of human neuronal NOS at 2.03 Å resolution is reported and a different crystal form of human endothelial NOS is reported at 1.73 Å resolution.
Keywords: nitric oxide synthase.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

