The crystal structure of human Argonaute2
- PMID: 22539551
- PMCID: PMC3521581
- DOI: 10.1126/science.1221551
The crystal structure of human Argonaute2
Abstract
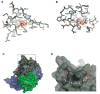
Argonaute proteins form the functional core of the RNA-induced silencing complexes that mediate RNA silencing in eukaryotes. The 2.3 angstrom resolution crystal structure of human Argonaute2 (Ago2) reveals a bilobed molecule with a central cleft for binding guide and target RNAs. Nucleotides 2 to 6 of a heterogeneous mixture of guide RNAs are positioned in an A-form conformation for base pairing with target messenger RNAs. Between nucleotides 6 and 7, there is a kink that may function in microRNA target recognition or release of sliced RNA products. Tandem tryptophan-binding pockets in the PIWI domain define a likely interaction surface for recruitment of glycine-tryptophan-182 (GW182) or other tryptophan-rich cofactors. These results will enable structure-based approaches for harnessing the untapped therapeutic potential of RNA silencing in humans.
Figures



Comment in
-
Biochemistry. Guided tour to the heart of RISC.Science. 2012 May 25;336(6084):985-6. doi: 10.1126/science.1223549. Science. 2012. PMID: 22628640 No abstract available.
References
-
- Liu J, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004 Sep 3;305:1437. - PubMed
-
- Meister G, et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004 Jul 23;15:185. - PubMed
-
- Braun JE, Huntzinger E, Fauser M, Izaurralde E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol Cell. 2011 Oct 7;44:120. - PubMed
-
- Fabian MR, et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat Struct Mol Biol. 2011 Nov;18:1211. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

