Structural mechanism of RuBisCO activation by carbamylation of the active site lysine
- PMID: 23112176
- PMCID: PMC3503183
- DOI: 10.1073/pnas.1210754109
Structural mechanism of RuBisCO activation by carbamylation of the active site lysine
Abstract
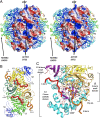
Ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO) is a crucial enzyme in carbon fixation and the most abundant protein on earth. It has been studied extensively by biochemical and structural methods; however, the most essential activation step has not yet been described. Here, we describe the mechanistic details of Lys carbamylation that leads to RuBisCO activation by atmospheric CO(2). We report two crystal structures of nitrosylated RuBisCO from the red algae Galdieria sulphuraria with O(2) and CO(2) bound at the active site. G. sulphuraria RuBisCO is inhibited by cysteine nitrosylation that results in trapping of these gaseous ligands. The structure with CO(2) defines an elusive, preactivation complex that contains a metal cation Mg(2+) surrounded by three H(2)O/OH molecules. Both structures suggest the mechanism for discriminating gaseous ligands by their quadrupole electric moments. We describe conformational changes that allow for intermittent binding of the metal ion required for activation. On the basis of these structures we propose the individual steps of the activation mechanism. Knowledge of all these elements is indispensable for engineering RuBisCO into a more efficient enzyme for crop enhancement or as a remedy to global warming.
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
-
- Ellis RJ. Most abundant protein in the world. Trends Biochem Sci. 1979;4(11):241–244.
-
- Andersson I. Catalysis and regulation in Rubisco. J Exp Bot. 2008;59(7):1555–1568. - PubMed
-
- Andersson I, Backlund A. Structure and function of Rubisco. Plant Physiol Biochem. 2008;46(3):275–291. - PubMed
-
- Ashida H, et al. RuBisCO-like proteins as the enolase enzyme in the methionine salvage pathway: Functional and evolutionary relationships between RuBisCO-like proteins and photosynthetic RuBisCO. J Exp Bot. 2008;59(7):1543–1554. - PubMed
-
- Lorimer GH, Badger MR, Andrews TJ. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976;15(3):529–536. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

