Structural insights into lipid-dependent reversible dimerization of human GLTP
- PMID: 23519669
- PMCID: PMC3606038
- DOI: 10.1107/S0907444913000024
Structural insights into lipid-dependent reversible dimerization of human GLTP
Abstract
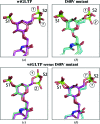
Human glycolipid transfer protein (hsGLTP) forms the prototypical GLTP fold and is characterized by a broad transfer selectivity for glycosphingolipids (GSLs). The GLTP mutation D48V near the `portal entrance' of the glycolipid binding site has recently been shown to enhance selectivity for sulfatides (SFs) containing a long acyl chain. Here, nine novel crystal structures of hsGLTP and the SF-selective mutant complexed with short-acyl-chain monoSF and diSF in different crystal forms are reported in order to elucidate the potential functional roles of lipid-mediated homodimerization. In all crystal forms, the hsGLTP-SF complexes displayed homodimeric structures supported by similarly organized intermolecular interactions. The dimerization interface always involved the lipid sphingosine chain, the protein C-terminus (C-end) and α-helices 6 and 2, but the D48V mutant displayed a `locked' dimer conformation compared with the hinge-like flexibility of wild-type dimers. Differences in contact angles, areas and residues at the dimer interfaces in the `flexible' and `locked' dimers revealed a potentially important role of the dimeric structure in the C-end conformation of hsGLTP and in the precise positioning of the key residue of the glycolipid recognition centre, His140. ΔY207 and ΔC-end deletion mutants, in which the C-end is shifted or truncated, showed an almost complete loss of transfer activity. The new structural insights suggest that ligand-dependent reversible dimerization plays a role in the function of human GLTP.
Keywords: GLTP fold; glycolipid transfer protein; lipid-mediated homodimerization; selectivity; sulfatides.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

