Antibacterial drug leads targeting isoprenoid biosynthesis
- PMID: 23248302
- PMCID: PMC3538244
- DOI: 10.1073/pnas.1219899110
Antibacterial drug leads targeting isoprenoid biosynthesis
Abstract
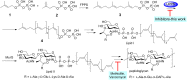
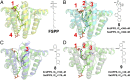
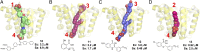
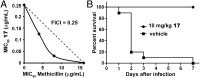
With the rise in resistance to antibiotics such as methicillin, there is a need for new drugs. We report here the discovery and X-ray crystallographic structures of 10 chemically diverse compounds (benzoic, diketo, and phosphonic acids, as well as a bisamidine and a bisamine) that inhibit bacterial undecaprenyl diphosphate synthase, an essential enzyme involved in cell wall biosynthesis. The inhibitors bind to one or more of the four undecaprenyl diphosphate synthase inhibitor binding sites identified previously, with the most active leads binding to site 4, outside the catalytic center. The most potent leads are active against Staphylococcus aureus [minimal inhibitory concentration (MIC)(90) ∼0.25 µg/mL], and one potently synergizes with methicillin (fractional inhibitory concentration index = 0.25) and is protective in a mouse infection model. These results provide numerous leads for antibacterial development and open up the possibility of restoring sensitivity to drugs such as methicillin, using combination therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

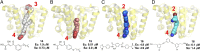

References
-
- Scholte AA, Eubanks LM, Poulter CD, Vederas JC. Synthesis and biological activity of isopentenyl diphosphate analogues. Bioorg Med Chem. 2004;12(4):763–770. - PubMed
-
- Guo RT, et al. Crystal structures of undecaprenyl pyrophosphate synthase in complex with magnesium, isopentenyl pyrophosphate, and farnesyl thiopyrophosphate: Roles of the metal ion and conserved residues in catalysis. J Biol Chem. 2005;280(21):20762–20774. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- GM08326/GM/NIGMS NIH HHS/United States
- U54 HD071600/HD/NICHD NIH HHS/United States
- T32 GM008326/GM/NIGMS NIH HHS/United States
- CA158191/CA/NCI NIH HHS/United States
- GM31749/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- GM65307/GM/NIGMS NIH HHS/United States
- AI074233/AI/NIAID NIH HHS/United States
- R01 GM065307/GM/NIGMS NIH HHS/United States
- HD071600/HD/NICHD NIH HHS/United States
- R01 GM031749/GM/NIGMS NIH HHS/United States
- R01 CA158191/CA/NCI NIH HHS/United States
- U54 AI057153/AI/NIAID NIH HHS/United States
- R01 AI074233/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

