Antitrypanosomal lead discovery: identification of a ligand-efficient inhibitor of Trypanosoma cruzi CYP51 and parasite growth
- PMID: 23448316
- PMCID: PMC3612894
- DOI: 10.1021/jm400012e
Antitrypanosomal lead discovery: identification of a ligand-efficient inhibitor of Trypanosoma cruzi CYP51 and parasite growth
Abstract
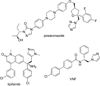
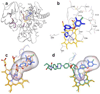
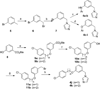
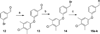
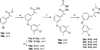
Chagas disease is caused by the intracellular protozoan parasite Trypanosomal cruzi , and current drugs are lacking in terms of desired safety and efficacy profiles. Following on a recently reported high-throughput screening campaign, we have explored initial structure-activity relationships around a class of imidazole-based compounds. This profiling has uncovered compounds 4c (NEU321) and 4j (NEU704), which are potent against in vitro cultures of T. cruzi and are greater than 160-fold selective over host cells. We report in vitro drug metabolism and properties profiling of 4c and show that this chemotype inhibits the T. cruzi CYP51 enzyme, an observation confirmed by X-ray crystallographic analysis. We compare the binding orientation of 4c to that of other, previously reported inhibitors. We show that 4c displays a significantly better ligand efficiency and a shorter synthetic route over previously disclosed CYP51 inhibitors, and should therefore be considered a promising lead compound for further optimization.
Figures
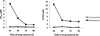


References
-
- World Health Organization; 2012. http://www.who.int/mediacentre/factsheets/fs340/en/index.html.
-
- Urbina JA, Payares G, Sanoja C, Molina J, Lira R, Brener Z, Romanha AJ. Parasitological cure of acute and chronic experimental Chagas disease using the long-acting experimental triazole TAK-187. Activity against drug-resistant Trypanosoma cruzi strains. Int J Antimicrob Agents. 2003;21:39–48. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

