Structures of a Na+-coupled, substrate-bound MATE multidrug transporter
- PMID: 23341609
- PMCID: PMC3568332
- DOI: 10.1073/pnas.1219901110
Structures of a Na+-coupled, substrate-bound MATE multidrug transporter
Abstract
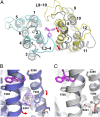
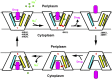
Multidrug transporters belonging to the multidrug and toxic compound extrusion (MATE) family expel dissimilar lipophilic and cationic drugs across cell membranes by dissipating a preexisting Na(+) or H(+) gradient. Despite its clinical relevance, the transport mechanism of MATE proteins remains poorly understood, largely owing to a lack of structural information on the substrate-bound transporter. Here we report crystal structures of a Na(+)-coupled MATE transporter NorM from Neisseria gonorrheae in complexes with three distinct translocation substrates (ethidium, rhodamine 6G, and tetraphenylphosphonium), as well as Cs(+) (a Na(+) congener), all captured in extracellular-facing and drug-bound states. The structures revealed a multidrug-binding cavity festooned with four negatively charged amino acids and surprisingly limited hydrophobic moieties, in stark contrast to the general belief that aromatic amino acids play a prominent role in multidrug recognition. Furthermore, we discovered an uncommon cation-π interaction in the Na(+)-binding site located outside the drug-binding cavity and validated the biological relevance of both the substrate- and cation-binding sites by conducting drug resistance and transport assays. Additionally, we uncovered potential rearrangement of at least two transmembrane helices upon Na(+)-induced drug export. Based on our structural and functional analyses, we suggest that Na(+) triggers multidrug extrusion by inducing protein conformational changes rather than by directly competing for the substrate-binding amino acids. This scenario is distinct from the canonical antiport mechanism, in which both substrate and counterion compete for a shared binding site in the transporter. Collectively, our findings provide an important step toward a detailed and mechanistic understanding of multidrug transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446(7137):749–757. - PubMed
-
- Brown MH, Paulsen IT, Skurray RA. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol. 1999;31(1):394–395. - PubMed
-
- Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci. 2006;27(11):587–593. - PubMed
-
- Kuroda T, Tsuchiya T. Multidrug efflux transporters in the MATE family. Biochim Biophys Acta. 2009;1794(5):763–768. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

